Metabolic Scar Assessment with18F-FDG PET: Correlation to Ischemic Ventricular Tachycardia Substrate and Successful Ablation Sites
- PMID: 33893186
- PMCID: PMC8612320
- DOI: 10.2967/jnumed.120.246413
Metabolic Scar Assessment with18F-FDG PET: Correlation to Ischemic Ventricular Tachycardia Substrate and Successful Ablation Sites
Abstract
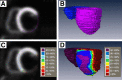
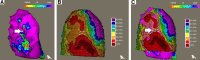
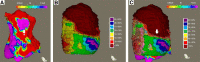
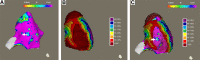
The functional and molecular imaging characteristics of ischemic ventricular tachycardia (VT) substrate are incompletely understood. Our objective was to compare regional 18F-FDG PET tracer uptake with detailed electroanatomic maps (EAMs) in a more extensive series of postinfarction VT patients to define the metabolic properties of VT substrate and successful ablation sites. Methods: Three-dimensional (3D) metabolic left ventricular reconstructions were created from perfusion-normalized 18F-FDG PET images in consecutive patients undergoing VT ablation. PET defects were classified as severe (defined as <50% uptake) or moderate (defined as 50%-70% uptake), as referenced to the maximal 17-segment uptake. Color-coded PET scar reconstructions were coregistered with corresponding high-resolution 3D EAMs, which were classified as indicating dense scarring (defined as voltage < 0.5 mV), normal myocardium (defined as voltage > 1.5 mV), or border zones (defined as voltage of 0.5-1.5 mV). Results: All 56 patients had ischemic cardiomyopathy (ejection fraction, 29% ± 12%). Severe PET defects were larger than dense scarring, at 63.0 ± 48.4 cm2 versus 13.8 ± 33.1 cm2 (P < 0.001). Similarly, moderate/severe PET defects (≤70%) were larger than areas with abnormal voltage (≤1.5 mV) measuring 105.1 ± 67.2 cm2 versus 56.2 ± 62.6 cm2 (P < 0.001). Analysis of bipolar voltage (23,389 mapping points) showed decreased voltage among severe PET defects (n = 10,364; 0.5 ± 0.3 mV) and moderate PET defects (n = 5,243; 1.5 ± 0.9 mV, P < 0.01), with normal voltage among normal PET areas (>70% uptake) (n = 7,782, 3.2 ± 1.3 mV, P < 0.001). Eighty-eight percent of VT channel or exit sites (n = 44) were metabolically abnormal (severe PET defect, 78%; moderate PET defect, 10%), whereas 12% (n = 6) were in PET-normal areas. Metabolic channels (n = 26) existed in 45% (n = 25) of patients, with an average length and width of 17.6 ± 12.5 mm and 10.3 ± 4.2 mm, respectively. Metabolic channels were oriented predominantly in the apex or base (86%), harboring VT channel or exit sites in 31%. Metabolic rapid-transition areas (>50% change in 18F-FDG tracer uptake/15 mm) were detected in 59% of cases (n = 33), colocalizing to VT channels or exit sites (15%) or near these sites (85%, 12.8 ± 8.5 mm). Metabolism-voltage mismatches in which there was a severe PET defect but voltage indicating normal myocardium were seen in 21% of patients (n = 12), 41% of whom were harboring VT channel or exit sites. Conclusion: Abnormal 18F-FDG uptake categories could be detected using incremental 3D step-up reconstructions. They predicted decreasing bipolar voltages and VT channel or exit sites in about 90% of cases. Additionally, functional imaging allowed detection of novel molecular tissue characteristics within the ischemic VT substrate such as metabolic channels, rapid-transition areas, and metabolism-voltage mismatches demonstrating intrasubstrate heterogeneity and providing possible targets for imaging-guided ablation.
Keywords: 18F-FDG PET imaging; VT channel or exit sites; functional imaging; ventricular tachycardia substrate.
© 2021 by the Society of Nuclear Medicine and Molecular Imaging.
Figures


References
-
- Morady F, Harvey M, Kalbfleisch SJ, et al. Radiofrequency catheter ablation of ventricular tachycardia in patients with coronary artery disease. Circulation. 1993;87:363–372. - PubMed
-
- Sacher F, Lim HS, Derval N, et al. Substrate mapping and ablation for ventricular tachycardia: the LAVA approach. J Cardiovasc Electrophysiol. 2015;26:464–471. - PubMed
-
- Marchlinski FE, Callans DJ, Gottlieb CD, et al. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and non-ischemic cardiomyopathy. Circulation. 2000;101:1288–1296. - PubMed
-
- Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter ThermoCool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–2782. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
