COVID-19 mRNA Vaccination: Age and Immune Status and Its Association with Axillary Lymph Node PET/CT Uptake
- PMID: 33893188
- PMCID: PMC8717182
- DOI: 10.2967/jnumed.121.262194
COVID-19 mRNA Vaccination: Age and Immune Status and Its Association with Axillary Lymph Node PET/CT Uptake
Abstract
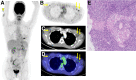
With hundreds of millions of coronavirus disease 2019 (COVID-19) messenger RNA (mRNA)-based vaccine doses planned to be delivered worldwide in the upcoming months, it is important to recognize PET/CT findings in recently vaccinated immunocompetent or immunocompromised patients. We aimed to assess PET/CT uptake in the deltoid muscle and axillary lymph nodes of patients who received a COVID-19 mRNA-based vaccine and to evaluate its association with patient age and immune status. Methods: All consecutive adults who underwent PET/CT scans with any radiotracer at our center during the first month of a national COVID-19 vaccination rollout (between December 23, 2020, and January 27, 2021) and had received the vaccination were included. Data on clinical status, laterality, and time from vaccination were prospectively collected, retrospectively analyzed, and correlated with deltoid muscle and axillary lymph node uptake. Results: Of 426 eligible subjects (median age, 67 ± 12 y; 49% female), 377 (88%) underwent PET/CT with 18F-FDG, and positive axillary lymph node uptake was seen in 45% of them. Multivariate logistic regression analysis revealed a strong inverse association between positive 18F-FDG uptake in ipsilateral lymph nodes and patient age (odds ratio [OR], 0.57; 95% CI, 0.45-0.72; P < 0.001), immunosuppressive treatment (OR, 0.37; 95% CI, 0.20-0.64; P = 0.003), and presence of hematologic disease (OR, 0.44; 95% CI, 0.24-0.8; P = 0.021). No such association was found for deltoid muscle uptake. The number of days from the last vaccination and the number of vaccine doses were also significantly associated with increased odds of positive lymph node uptake. Conclusion: After mRNA-based COVID-19 vaccination, a high proportion of patients showed ipsilateral lymph node axillary uptake, which was more common in immunocompetent patients. This information will help with the recognition of PET/CT pitfalls and may hint about the patient's immune response to the vaccine.
Keywords: COVID-19; PET/CT; axillary lymphadenopathy; immunogenicity; mRNA vaccine.
© 2022 by the Society of Nuclear Medicine and Molecular Imaging.
Figures




References
-
- WHO coronavirus (COVID-19) dashboard. World Health Organization website. https://covid19.who.int. Updated October 5, 2021. Accessed October 6, 2021.
-
- Comirnaty and Pfizer-BioNTech COVID-19 vaccine. U.S. Food and Drug Administration website. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise.... Updated September 24, 2021. Accessed February 5, 2021.
-
- Moderna COVID-19 vaccine. U.S. Food and Drug Administration website. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise.... Updated August 31, 2021. Accessed February 5, 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
