Co-delivery of IOX1 and doxorubicin for antibody-independent cancer chemo-immunotherapy
- PMID: 33893275
- PMCID: PMC8065121
- DOI: 10.1038/s41467-021-22407-6
Co-delivery of IOX1 and doxorubicin for antibody-independent cancer chemo-immunotherapy
Abstract
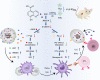
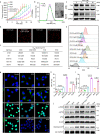
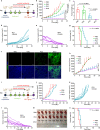
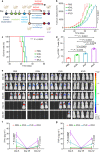
Anti-programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) antibodies are currently used in the clinic to interupt the PD-1/PD-L1 immune checkpoint, which reverses T cell dysfunction/exhaustion and shows success in treating cancer. Here, we report a histone demethylase inhibitor, 5-carboxy-8-hydroxyquinoline (IOX1), which inhibits tumour histone demethylase Jumonji domain-containing 1A (JMJD1A) and thus downregulates its downstream β-catenin and subsequent PD-L1, providing an antibody-independent paradigm interrupting the PD-1/PD-L1 checkpoint. Synergistically, IOX1 inhibits cancer cells' P-glycoproteins (P-gp) through the JMJD1A/β-catenin/P-gp pathway and greatly enhances doxorubicin (DOX)-induced immune-stimulatory immunogenic cell death. As a result, the IOX1 and DOX combination greatly promotes T cell infiltration and activity and significantly reduces tumour immunosuppressive factors. Their liposomal combination reduces the growth of various murine tumours, including subcutaneous, orthotopic, and lung metastasis tumours, and offers a long-term immunological memory function against tumour rechallenging. This work provides a small molecule-based potent cancer chemo-immunotherapy.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Blocking of the PD-1/PD-L1 interaction by a novel cyclic peptide inhibitor for cancer immunotherapy.Sci China Life Sci. 2021 Apr;64(4):548-562. doi: 10.1007/s11427-020-1740-8. Epub 2020 Jul 28. Sci China Life Sci. 2021. PMID: 32737851
-
Design of an Injectable Polypeptide Hydrogel Depot Containing the Immune Checkpoint Blocker Anti-PD-L1 and Doxorubicin to Enhance Antitumor Combination Therapy.Macromol Biosci. 2021 Jun;21(6):e2100049. doi: 10.1002/mabi.202100049. Epub 2021 Apr 19. Macromol Biosci. 2021. PMID: 33871152
-
PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations.Hum Vaccin Immunother. 2019;15(5):1111-1122. doi: 10.1080/21645515.2019.1571892. Epub 2019 Mar 19. Hum Vaccin Immunother. 2019. PMID: 30888929 Free PMC article. Review.
-
Immune Checkpoint Inhibition Followed by Tumor Infiltration of Dendritic Cells in Murine Neuro-2a Neuroblastoma.J Surg Res. 2020 Sep;253:201-213. doi: 10.1016/j.jss.2020.03.059. Epub 2020 May 4. J Surg Res. 2020. PMID: 32380346
-
T cell checkpoint regulators in the heart.Cardiovasc Res. 2019 Apr 15;115(5):869-877. doi: 10.1093/cvr/cvz025. Cardiovasc Res. 2019. PMID: 30721928 Free PMC article. Review.
Cited by
-
Mitochondria-Targeted Multifunctional Nanoparticles Combine Cuproptosis and Programmed Cell Death-1 Downregulation for Cancer Immunotherapy.Adv Sci (Weinh). 2024 Sep;11(35):e2403520. doi: 10.1002/advs.202403520. Epub 2024 Jul 16. Adv Sci (Weinh). 2024. PMID: 39013093 Free PMC article.
-
Engineered nanomedicines block the PD-1/PD-L1 axis for potentiated cancer immunotherapy.Acta Pharmacol Sin. 2022 Nov;43(11):2749-2758. doi: 10.1038/s41401-022-00910-w. Epub 2022 Apr 28. Acta Pharmacol Sin. 2022. PMID: 35484402 Free PMC article. Review.
-
Nanoscale coordination polymer synergizes photodynamic therapy and toll-like receptor activation for enhanced antigen presentation and antitumor immunity.Biomaterials. 2023 Nov;302:122334. doi: 10.1016/j.biomaterials.2023.122334. Epub 2023 Sep 22. Biomaterials. 2023. PMID: 37776767 Free PMC article.
-
Histone demethylase JMJD1A in cancer progression and therapeutic resistance.Mol Carcinog. 2022 Apr;61(4):392-396. doi: 10.1002/mc.23390. Epub 2022 Jan 12. Mol Carcinog. 2022. PMID: 35020958 Free PMC article.
-
A landscape of recent advances in lipid nanoparticles and their translational potential for the treatment of solid tumors.Bioeng Transl Med. 2023 Nov 9;9(2):e10601. doi: 10.1002/btm2.10601. eCollection 2024 Mar. Bioeng Transl Med. 2023. PMID: 38435821 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

