Stretchable chiral pockets for palladium-catalyzed highly chemo- and enantioselective allenylation
- PMID: 33893276
- PMCID: PMC8065118
- DOI: 10.1038/s41467-021-22498-1
Stretchable chiral pockets for palladium-catalyzed highly chemo- and enantioselective allenylation
Abstract
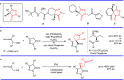
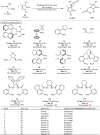
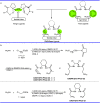
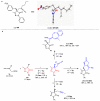
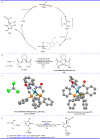
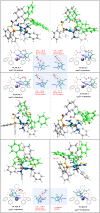
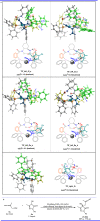
Pyrazolones are a vital class of heterocycles possessing various biological properties and much attention is paid to the diversified synthesis of enantiopure pyrazolone derivatives. We describe here the development of diphenylphosphinoalkanoic acid based chiral bisphosphine ligands, which are successfully applied to the palladium-catalyzed asymmetric allenylation of racemic pyrazol-5-ones. The reaction affords C-allenylation products, optically active pyrazol-5-ones bearing an allene unit, in high chemo- and enantioselectivity, with DACH-ZYC-Phos-C1 as the best ligand. The synthetic potential of the C-allenylation products is demonstrated. Furthermore, the enantioselectivity observed with DACH-ZYC-Phos-C1 is rationalized by density functional theory studies.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Varvounis, G. Advances in Heterocyclic Chemistry (ed. Katritzky, A. R.) Ch. 2 (Elsevier Inc., 2009).
-
- Chumakova, L. et al. Compounds Useful as Modulators of TRPM8. US0376136 (2015).
-
- Zhang, Y. et al. Divergent cascade construction of skeletally diverse “Privileged” pyrazole-derived molecular architectures. Eur. J. Org. Chem. 2030–2037 (2015).
Publication types
LinkOut - more resources
Full Text Sources

