Application of circulating genetically abnormal cells in the diagnosis of early-stage lung cancer
- PMID: 33893839
- PMCID: PMC11801133
- DOI: 10.1007/s00432-021-03648-w
Application of circulating genetically abnormal cells in the diagnosis of early-stage lung cancer
Abstract
Purpose: Lung cancer is the leading cause of cancer-related death worldwide. The early detection of lung cancer is crucial for the diagnosis of this disease. Therefore, an effective and noninvasive method for the early diagnosis of lung cancer is urgently needed.
Methods: To evaluate the diagnostic performance of circulating genetically abnormal cells (CACs) in early lung cancer, a total of 63 participants who completed CAC detection by Zhuhai SanMed Biotech Inc. and obtained pathological results from January to December 2020 were included in our study; 50 patients had lung cancer and 13 patients had benign lung disease. The levels of lung cancer-related markers in peripheral blood and the chest computed tomography (CT) imaging characteristics of these patients were collected before pathological acquisition.
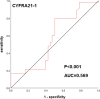
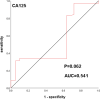
Results: The positive rate of CAC was 90.0% in the lung cancer group and 23.1% in the benign lung disease group, and the difference was statistically significant (P < 0.01). The area under the receiver operating characteristic (ROC) curve of CAC was 0.837, the sensitivity was 90%, and the specificity was 76.9%. The area under the ROC curve and sensitivity were both higher than those of the combined or single serum tumor marker test.
Conclusions: This study preliminarily concludes that the CAC test, as a noninvasive test, has high sensitivity and specificity for the early diagnosis of lung cancer. This test is expected to help with the early detection of disease in lung cancer patients.
Keywords: CAC; Circulating genetically abnormal cells; Early diagnosis; Lung cancer.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
There is no conflict of interest involved in this study.
Figures






References
-
- Bade BC, Dela Cruz CS (2020) Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med 41:1–24. 10.1016/j.ccm.2019.10.001 - PubMed
-
- Blackhall F, Frese KK, Simpson K, Kilgour E, Brady G, Dive C (2018) Will liquid biopsies improve outcomes for patients with small-cell lung cancer? Lancet Oncol 19:e470–e481. 10.1016/s1470-2045(18)30455-8 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

