Manganese-Catalyzed Hydroborations with Broad Scope
- PMID: 33894033
- PMCID: PMC8362021
- DOI: 10.1002/anie.202103550
Manganese-Catalyzed Hydroborations with Broad Scope
Abstract
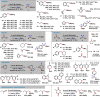
Reductive transformations of easily available oxidized matter are at the heart of synthetic manipulation and chemical valorization. The applications of catalytic hydrofunctionalization benefit from the use of liquid reducing agents and operationally facile setups. Metal-catalyzed hydroborations provide a highly prolific platform for reductive valorizations of stable C=X electrophiles. Here, we report an especially facile, broad-scope reduction of various functions including carbonyls, carboxylates, pyridines, carbodiimides, and carbonates under very mild conditions with the inexpensive pre-catalyst Mn(hmds)2 . The reaction could be successfully applied to depolymerizations.
Keywords: amides; carbon dioxide; carbonates; depolymerization; hydroboration; manganese.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- None
-
- The Handbook of Homogeneous Hydrogenation (Eds.: de Vries J. G., Elsevier C. J.), Wiley-VCH, Weinheim, 2007;
-
- Nishimura S., Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, Wiley, New York, 2001;
-
- Catalysis without Precious Metals (Ed.: Bullock R. M.), Wiley-VCH, Weinheim, 2010;
-
- Non-Noble Metal Catalysis: Molecular Approaches and Reactions (Ed.: Klein Gebbink R. J. M., Moret M.-E.), Wiley-VCH, Weinheim, 2019;
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

