Rethinking neutrophils and eosinophils in chronic rhinosinusitis
- PMID: 33895002
- PMCID: PMC8355033
- DOI: 10.1016/j.jaci.2021.03.024
Rethinking neutrophils and eosinophils in chronic rhinosinusitis
Abstract
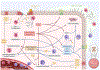
Chronic rhinosinusitis (CRS) often is characterized by an eosinophilic inflammatory pattern, nowadays referred to as type 2 inflammation, although the mucosal inflammation is dominated by neutrophils in about a third of the patients. Neutrophils are typically predominant in 50% of patients with CRS without nasal polyps, but also are found to play a role in patients with severe type 2 CRS with nasal polyp disease. This review aims at summarizing the current understanding of the eosinophilic and neutrophilic inflammation in CRS pathophysiology, and provides a discussion of their reciprocal interactions and the clinical impact of the mixed presentation in patients with severe type 2 CRS with nasal polyps. A solid understanding of these interactions is of utmost importance when treating uncontrolled severe CRS with nasal polyps with biologicals that are preferentially directed toward type 2 inflammation. We here focus on recent findings on both eosinophilic and neutrophilic granulocytes, their subgroups and the activation status, and their interactions in CRS.
Keywords: Charcot-Leyden crystals; Chronic rhinosinusitis; IL-17; activation; biologicals; eosinophils; extracellular traps; neutrophils; type 2 inflammation.
Copyright © 2021 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
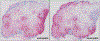
References
-
- Khan A, Vandeplas G, Huynh TMT, Joish VN, Mannent L, Tomassen P, et al. The Global Allergy and Asthma European Network (GALEN rhinosinusitis cohort: a large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57(1):32–42. - PubMed
-
- Bachert C, Han JK, Wagenmann M, Hosemann W, Lee SE, Backer V, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: Definitions and management. J Allergy Clin Immunol. 2021;147(1):29–36.
-
- Bachert C, Zhang N, Hellings PW, Bousquet J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141(5):1543–51. - PubMed
-
- Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61(11):1280–9. - PubMed
-
- Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449–56 e4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

