Spatial scales in human movement between reservoirs of infection
- PMID: 33895180
- PMCID: PMC8204271
- DOI: 10.1016/j.jtbi.2021.110726
Spatial scales in human movement between reservoirs of infection
Abstract
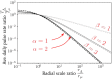
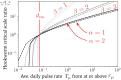
The life cycle of parasitic organisms that are the cause of much morbidity in humans often depend on reservoirs of infection for transmission into their hosts. Understanding the daily, monthly and yearly movement patterns of individuals between reservoirs is therefore of great importance to implementers of control policies seeking to eliminate various parasitic diseases as a public health problem. This is due to the fact that the underlying spatial extent of the reservoir of infection, which drives transmission, can be strongly affected by inputs from external sources, i.e., individuals who are not spatially attributed to the region defined by the reservoir itself can still migrate and contribute to it. In order to study the importance of these effects, we build and examine a novel theoretical model of human movement between spatially-distributed focal points for infection clustered into regions defined as 'reservoirs of infection'. Using our model, we vary the spatial scale of human moment defined around focal points and explicitly calculate how varying this definition can influence the temporal stability of the effective transmission dynamics - an effect which should strongly influence how control measures, e.g., mass drug administration (MDA), define evaluation units (EUs). Considering the helminth parasites as our main example, by varying the spatial scale of human movement, we demonstrate that a critical scale exists around infectious focal points at which the migration rate into their associated reservoir can be neglected for practical purposes. This scale varies by species and geographic region, but is generalisable as a concept to infectious reservoirs of varying spatial extents and shapes. Our model is designed to be applicable to a very general pattern of infectious disease transmission modified by the migration of infected individuals between clustered communities. In particular, it may be readily used to study the spatial structure of hosts for macroparasites with temporally stationary distributions of infectious focal point locations over the timescales of interest, which is viable for the soil-transmitted helminths and schistosomes. Additional developments will be necessary to consider diseases with moving reservoirs, such as vector-born filarial worm diseases.
Keywords: Control policies; Mathematical models; Monitoring and evaluation; Spatial infection model.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Anderson R.M., May R.M. Population dynamics of human helminth infections: control by chemotherapy. Nature. 1982;297(5867):557. - PubMed
-
- Anderson, R., and May, R. 1992. Infectious Diseases of Humans: Dynamics and Control, Dynamics and Control, OUP Oxford, 1992. URL https://books.google.co.uk/books?id=HT0-xXBguQC.
-
- Ásbjörnsdóttir, K. H., Ajjampur, S. S. R., Anderson, R. M., Bailey, R., Gardiner, I., Halliday, K. E., Ibikounle, M., Kalua, K., Kang, G., Littlewood, D. T. J., Luty, A. J. F., Means, A. R., Oswald, W., Pullan, R. L., Sarkar, R., Schr, F., Szpiro, A., Truscott, J. E., Werkman, M., Yard, E., Walson, J. L., Team, T. D. T., 2018. Assessing the feasibility of interrupting the transmission of soil-transmitted helminths through mass drug administration: The deworm3 cluster randomized trial protocol, PLOS Neglected Trop. Diseases 12;1:1–16. https://doi.org/10.1371/journal.pntd.0006166. - DOI - PMC - PubMed
-
- Brooker, S., Bethony, J., Hotez, P. J. 2004. Human hookworm infection in the 21st century, Vol. 58 of Advances in Parasitology, Academic Press, 2004, pp. 197–288. https://doi.org/10.1016/S0065-308X(04)58004-1. http://www.sciencedirect.com/science/article/pii/S0065308X04580041. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

