Reconstructing the heart using iPSCs: Engineering strategies and applications
- PMID: 33895197
- PMCID: PMC8378256
- DOI: 10.1016/j.yjmcc.2021.04.006
Reconstructing the heart using iPSCs: Engineering strategies and applications
Abstract
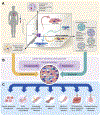
Induced pluripotent stem cells (iPSCs) have emerged as a key component of cardiac tissue engineering, enabling studies of cardiovascular disease mechanisms, drug responses, and developmental processes in human 3D tissue models assembled from isogenic cells. Since the very first engineered heart tissues were introduced more than two decades ago, a wide array of iPSC-derived cardiac spheroids, organoids, and heart-on-a-chip models have been developed incorporating the latest available technologies and materials. In this review, we will first outline the fundamental biological building blocks required to form a functional unit of cardiac muscle, including iPSC-derived cells differentiated by soluble factors (e.g., small molecules), extracellular matrix scaffolds, and exogenous biophysical maturation cues. We will then summarize the different fabrication approaches and strategies employed to reconstruct the heart in vitro at varying scales and geometries. Finally, we will discuss how these platforms, with continued improvements in scalability and tissue maturity, can contribute to both basic cardiovascular research and clinical applications in the future.
Keywords: 3D bioprinting; Cardiac organoids; Cardiovascular tissue engineering; Engineered heart tissue; iPSC.
Copyright © 2021. Published by Elsevier Ltd.
Figures

References
-
- Heisenberg CP, Bellaiche Y, Forces in tissue morphogenesis and patterning, Cell 153 (2013) 948–962. - PubMed
-
- Srivastava D, Olson EN, A genetic blueprint for cardiac development, Nature 407 (2000) 221–226. - PubMed
-
- Epstein JA, Franklin h. Epstein lecture. Cardiac development and implications for heart disease, N. Engl. J. Med 363 (2010) 1638–1647. - PubMed
-
- Weinberger F, Mannhardt I, Eschenhagen T, Engineering cardiac muscle tissue: a maturating field of research, Circ. Res 120 (2017) 1487–1500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

