Next generation strategies for preventing preterm birth
- PMID: 33895215
- PMCID: PMC8217279
- DOI: 10.1016/j.addr.2021.04.021
Next generation strategies for preventing preterm birth
Abstract
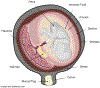
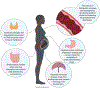
Preterm birth (PTB) is defined as delivery before 37 weeks of gestation. Globally, 15 million infants are born prematurely, putting these children at an increased risk of mortality and lifelong health challenges. Currently in the U.S., there is only one FDA approved therapy for the prevention of preterm birth. Makena is an intramuscular progestin injection given to women who have experienced a premature delivery in the past. Recently, however, Makena failed a confirmatory trial, resulting the Center for Drug Evaluation and Research's (CDER) recommendation for the FDA to withdrawal Makena's approval. This recommendation would leave clinicians with no therapeutic options for preventing PTB. Here, we outline recent interdisciplinary efforts involving physicians, pharmacologists, biologists, chemists, and engineers to understand risk factors associated with PTB, to define mechanisms that contribute to PTB, and to develop next generation therapies for preventing PTB. These advances have the potential to better identify women at risk for PTB, prevent the onset of premature labor, and, ultimately, save infant lives.
Keywords: Drug delivery; Nanomedicine; Nanoparticles; Pregnancy; Preterm birth; Vaginal drug delivery.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures





Similar articles
-
A chronicle of the 17-alpha hydroxyprogesterone caproate story to prevent recurrent preterm birth.Am J Obstet Gynecol. 2021 Feb;224(2):175-186. doi: 10.1016/j.ajog.2020.09.045. Epub 2020 Oct 6. Am J Obstet Gynecol. 2021. PMID: 33035472
-
Utilization of hydroxyprogesterone caproate among pregnancies with live birth deliveries in the sentinel distributed database.J Matern Fetal Neonatal Med. 2022 Dec;35(25):6291-6296. doi: 10.1080/14767058.2021.1910669. Epub 2021 Apr 29. J Matern Fetal Neonatal Med. 2022. PMID: 33926341
-
Rationale for current and future progestin-based therapies to prevent preterm birth.Best Pract Res Clin Obstet Gynaecol. 2018 Oct;52:114-125. doi: 10.1016/j.bpobgyn.2018.03.008. Epub 2018 Apr 11. Best Pract Res Clin Obstet Gynaecol. 2018. PMID: 29724668 Review.
-
FDA approved vs. Pharmacy compounded 17-OHPC-current issues for obstetricians to consider in reducing recurrent preterm birth.Curr Med Res Opin. 2020 Aug;36(8):1393-1401. doi: 10.1080/03007995.2020.1783220. Epub 2020 Jun 30. Curr Med Res Opin. 2020. PMID: 32544354
-
Efficacy of progesterone for prevention of preterm birth.Best Pract Res Clin Obstet Gynaecol. 2018 Oct;52:126-136. doi: 10.1016/j.bpobgyn.2018.08.006. Epub 2018 Sep 8. Best Pract Res Clin Obstet Gynaecol. 2018. PMID: 30266582 Review.
Cited by
-
A Progesterone Microneedle Patch for Self-Administration in the Prevention of Preterm Birth in a Mouse Model.Drug Des Devel Ther. 2025 Apr 1;19:2473-2490. doi: 10.2147/DDDT.S502701. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40190812 Free PMC article.
-
Improving the safety of N,N-dimethylacetamide (DMA) as a potential treatment for preterm birth in a pregnant mouse model using a vaginal nanoformulation.Biochim Biophys Acta Mol Basis Dis. 2025 Jun;1871(5):167822. doi: 10.1016/j.bbadis.2025.167822. Epub 2025 Mar 31. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40174791
-
In vitro and ex vivo models for evaluating vaginal drug delivery systems.Adv Drug Deliv Rev. 2022 Dec;191:114543. doi: 10.1016/j.addr.2022.114543. Epub 2022 Oct 5. Adv Drug Deliv Rev. 2022. PMID: 36208729 Free PMC article. Review.
-
Improving the Safety of N,N-Dimethylacetamide (DMA) as a Potential Treatment for Preterm Birth in a Pregnant Mouse Model Using a Vaginal Nanoformulation.bioRxiv [Preprint]. 2025 Jan 20:2025.01.16.633348. doi: 10.1101/2025.01.16.633348. bioRxiv. 2025. Update in: Biochim Biophys Acta Mol Basis Dis. 2025 Jun;1871(5):167822. doi: 10.1016/j.bbadis.2025.167822. PMID: 39896642 Free PMC article. Updated. Preprint.
-
Insulin-like growth factor-1 effects on kidney development in preterm piglets.Pediatr Res. 2024 Dec;96(7):1655-1665. doi: 10.1038/s41390-024-03222-3. Epub 2024 May 18. Pediatr Res. 2024. PMID: 38762663 Free PMC article.
References
-
- Cunningham F. Gary, L KJ, Bloom Steven L., Dashe Jodi S., Hoffman Barbara L., Casey Brian M., Spong Catherine Y.. (2018) Williams Obstetrics, Twenty-Fifth Edition, 25 edn., New York: McGraw-Hill.
-
- CDC (2019) Infant Mortality. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortal..., (accessed 09/17 2019).
-
- Dimes M.o., 2020 MARCH OF DIMES REPORT CARD, marchofdimes.org/reportcard, 2020.
-
- CDC (2019) Preterm Birth. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth..., (accessed 09/17/2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

