The TIM3/Gal9 signaling pathway: An emerging target for cancer immunotherapy
- PMID: 33895262
- PMCID: PMC8168453
- DOI: 10.1016/j.canlet.2021.04.011
The TIM3/Gal9 signaling pathway: An emerging target for cancer immunotherapy
Abstract
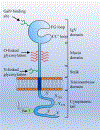
Immune checkpoint blockade has shown unprecedented and durable clinical response in a wide range of cancers. T cell immunoglobulin and mucin domain 3 (TIM3) is an inhibitory checkpoint protein that is highly expressed in tumor-infiltrating lymphocytes. In various cancers, the interaction of TIM3 and Galectin 9 (Gal9) suppresses anti-tumor immunity mediated by innate as well as adaptive immune cells. Thus, the blockade of the TIM3/Gal9 interaction is a promising therapeutic approach for cancer therapy. In addition, co-blockade of the TIM3/Gal9 pathway along with the PD-1/PD-L1 pathway increases the therapeutic efficacy by overcoming non-redundant immune resistance induced by each checkpoint. Here, we summarize the physiological roles of the TIM3/Gal9 pathway in adaptive and innate immune systems. We highlight the recent clinical and preclinical studies showing the involvement of the TIM3/Gal9 pathway in various solid and blood cancers. In addition, we discuss the potential of using TIM3 and Gal9 as prognostic and predictive biomarkers in different cancers. An in-depth mechanistic understanding of the blockade of the TIM3/Gal9 signaling pathway in cancer could help in identifying patients who respond to this therapy as well as designing combination therapies.
Keywords: Immune checkpoint blockade; Immune checkpoint receptors; Immune suppression; Immuno-oncology; TIM3/Gal9.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no competing interests.
Declaration of interests
⊠The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK, The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity, Nat Immunol, 6 (2005) 1245–1252. - PubMed
-
- Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M, Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1, Nat Immunol, 13 (2012) 832–842. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

