Immunogenicity of a new gorilla adenovirus vaccine candidate for COVID-19
- PMID: 33895322
- PMCID: PMC8062434
- DOI: 10.1016/j.ymthe.2021.04.022
Immunogenicity of a new gorilla adenovirus vaccine candidate for COVID-19
Abstract
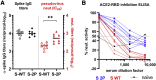
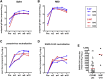
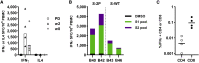
The coronavirus disease 2019 (COVID-19) pandemic caused by the emergent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) threatens global public health, and there is an urgent need to develop safe and effective vaccines. Here, we report the generation and the preclinical evaluation of a novel replication-defective gorilla adenovirus-vectored vaccine encoding the pre-fusion stabilized Spike (S) protein of SARS-CoV-2. We show that our vaccine candidate, GRAd-COV2, is highly immunogenic both in mice and macaques, eliciting both functional antibodies that neutralize SARS-CoV-2 infection and block Spike protein binding to the ACE2 receptor, and a robust, T helper (Th)1-dominated cellular response. We show here that the pre-fusion stabilized Spike antigen is superior to the wild type in inducing ACE2-interfering, SARS-CoV-2-neutralizing antibodies. To face the unprecedented need for vaccine manufacturing at a massive scale, different GRAd genome deletions were compared to select the vector backbone showing the highest productivity in stirred tank bioreactors. This preliminary dataset identified GRAd-COV2 as a potential COVID-19 vaccine candidate, supporting the translation of the GRAd-COV2 vaccine in a currently ongoing phase I clinical trial (ClinicalTrials.gov: NCT04528641).
Keywords: COVID-19; SARS-CoV-2; gorilla adenovirus; immunogenicity; vaccine.
Copyright © 2021 The American Society of Gene and Cell Therapy. All rights reserved.
Conflict of interest statement
Declaration of interests S. Capone, A.R., M.G., S.B., A.S., A.M.C., R.S., F.B., A. Leuzzi, E.L., G. Miselli, A.N., M.F., F.T., M.S., A.F., S. Colloca, and A.V. are employees of ReiThera Srl. A.F. and S. Colloca are also shareholders of Keires AG. A. Lahm is a consultant for ReiThera Srl. S. Colloca, A. Lahm, A.R., and A.V. are inventors of the patent application number 20183515.4, titled “Gorilla Adenovirus Nucleic Acid- and Amino Acid-Sequences, Vectors Containing Same, and Uses Thereof.” The other authors declare no competing interests.
Figures


References
-
- Vitelli A., Folgori A., Scarselli E., Colloca S., Capone S., Nicosia A. Chimpanzee adenoviral vectors as vaccines - challenges to move the technology into the fast lane. Expert Rev. Vaccines. 2017;16:1241–1252. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

