Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008-2017 ESME cohort
- PMID: 33895695
- PMCID: PMC8095121
- DOI: 10.1016/j.esmoop.2021.100114
Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008-2017 ESME cohort
Abstract
Background: Treatment strategies for metastatic breast cancer (MBC) have made great strides over the past 10 years. Real-world data allow us to evaluate the actual benefit of new treatments. ESME (Epidemio-Strategy-Medico-Economical)-MBC, a nationwide observational cohort (NCT03275311), gathers data of all consecutive MBC patients who initiated their treatment in 18 French Cancer Centres since 2008.
Patients and methods: We evaluated overall survival (OS) in the whole cohort (N = 20 446) and among subtypes: hormone receptor positive, human epidermal growth factor 2 negative (HR+/HER2-; N = 13 590), HER2+ (N = 3919), and triple-negative breast cancer (TNBC; N = 2937). We performed multivariable analyses including year of MBC diagnosis as one of the covariates, to assess the potential OS improvement over time, and we described exposure to newly released drugs at any time during MBC history by year of diagnosis (YOD).
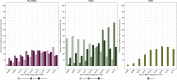
Results: The median follow-up of the whole cohort was 65.5 months (95% CI 64.6-66.7). Year of metastatic diagnosis appears as a strong independent prognostic factor for OS [Year 2016 HR 0.89 (95% CI 0.82-0.97); P = 0.009, using 2008 as reference]. This effect is driven by the HER2+ subcohort, where it is dramatic [Year 2016 HR 0.52 (95% CI 0.42-0.66); P < 0.001, using 2008 as reference]. YOD had, however, no sustained impact on OS among patients with TNBC [Year 2016 HR 0.93 (95% CI 0.77-1.11); P = 0.41, using 2008 as reference] nor among those with HR+/HER2- MBC [Year 2016 HR 1.02 (95% CI 0.91-1.13); P = 0.41, using 2008 as reference]. While exposure to newly released anti-HER2 therapies appeared very high (e.g. >70% of patients received pertuzumab from 2016 onwards), use of everolimus or eribulin was recorded in less than one-third of HR+/HER2- and TNBC cohorts, respectively, whatever YOD.
Conclusion: OS has dramatically improved among HER2+ MBC patients, probably in association with the release of several major HER2-directed therapies, whose penetrance was high. This trend was not observed in the other subtypes, but the impact of CDK4/6 inhibitors cannot yet be assessed.
Keywords: HER2; metastatic breast cancer; new drugs; overall survival; real-life.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure AG reports nonfinancial support from AstraZeneca, Roche, Pfizer, and Novartis, outside the submitted work. WJ reports grants, personal fees, and nonfinancial support from Astra Zeneca; personal fees and nonfinancial support from Eisai, Novartis, Roche, Pfizer, Eli Lilly, and Chugai; personal fees from MSD and BMS, outside the submitted work. P-HC reports grants and nonfinancial support from Roche and Pfizer, outside the submitted work. FC reports grants and personal fees from Merck Serono and BMS; nonfinancial support from Lilly; personal fees and nonfinancial support from Roche; and grants from AstraZeneca, outside the submitted work. ED reports personal fees and nonfinancial support from Novartis and Pfizer; and nonfinancial support from Lilly, outside the submitted work. TB reports personal fees and nonfinancial support from Roche and AstraZeneca; grants, personal fees, and nonfinancial support from Novartis and Pfizer; and personal fees from Seattle Genetics, outside the submitted work. DP reports personal fees from Laboratoire Roche, Pierre Fabre, Novartis, BMS, Eli-Lilly, Ipsen, MSD; personal fees and nonfinancial support from Laboratoire AstraZeneca; grants from Laboratoire MDS Avenir outside the submitted work and Laboratoire Roche (ESME program). SD reports grants and nonfinancial support from Pfizer, AstraZeneca, and Roche Genentech; grants from Novartis, Lilly, Puma, Myriad, Orion, Amgen, Sanofi, Genomic Health, GE, Servier, MSD, BMS, and Pierre Fabre, outside the submitted work. All remaining authors have nothing to disclose.
Figures


References
-
- Ferlay J., Colombet M., Soerjomataram I. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. - PubMed
-
- Cardoso F., Spence D., Mertz S. Global analysis of advanced/metastatic breast cancer: decade report (2005-2015) Breast. 2018;39:131–138. - PubMed
-
- Perou C.M., Sørlie T., Eisen M.B. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. - PubMed
-
- Swain S.M., Miles D., Kim S.-B. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. - PubMed
-
- Di V., Miles D., Verma S. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

