Issues and limitations of available biomarkers for fluoropyrimidine-based chemotherapy toxicity, a narrative review of the literature
- PMID: 33895696
- PMCID: PMC8095125
- DOI: 10.1016/j.esmoop.2021.100125
Issues and limitations of available biomarkers for fluoropyrimidine-based chemotherapy toxicity, a narrative review of the literature
Abstract
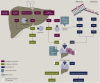
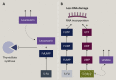
Fluoropyrimidine-based chemotherapies are widely used to treat gastrointestinal tract, head and neck, and breast carcinomas. Severe toxicities mostly impact rapidly dividing cell lines and can occur due to the partial or complete deficiency in dihydropyrimidine dehydrogenase (DPD) catabolism. Since April 2020, the European Medicines Agency (EMA) recommends DPD testing before any fluoropyrimidine-based treatment. Currently, different assays are used to predict DPD deficiency; the two main approaches consist of either phenotyping the enzyme activity (directly or indirectly) or genotyping the four main deficiency-related polymorphisms associated with 5-fluorouracil (5-FU) toxicity. In this review, we focused on the advantages and limitations of these diagnostic methods: direct phenotyping by evaluation of peripheral mononuclear cell DPD activity (PBMC-DPD activity), indirect phenotyping assessed by uracil levels or UH2/U ratio, and genotyping DPD of four variants directly associated with 5-FU toxicity. The risk of 5-FU toxicity increases with uracil concentration. Having a pyrimidine-related structure, 5-FU is catabolised by the same physiological pathway. By assessing uracil concentration in plasma, indirect phenotyping of DPD is then measured. With this approach, in France, a decreased 5-FU dose is systematically recommended at a uracil concentration of 16 ng/ml, which may lead to chemotherapy under-exposure as uracil concentration is a continuous variable and the association between uracil levels and DPD activity is not clear. We aim herein to describe the different available strategies developed to improve fluoropyrimidine-based chemotherapy safety, how they are implemented in routine clinical practice, and the possible relationship with inefficacy mechanisms.
Keywords: 5-FU; dihydropyrimidine dehydrogenase; genotype; phenotype; toxicity; uracil.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure The authors have declared no conflicts of interest.
Figures
References
-
- Screening for dihydropyrimidine dehydrogenase deficiency to prevent fluoropyrimidines (5-fluorouracil) severe adverse reactions, Recommendations, INCa, HAS, December 2018. https://www.has-sante.fr Available at:
-
- Heggie G.D., Sommadossi J.-P., Cross D.S., Huster W.J., Diasio R.B. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987;47(8):2203–2206. - PubMed
-
- Madi A., Fisher D., Maughan T.S. Pharmacogenetic analyses of 2183 patients with advanced colorectal cancer; potential role for common dihydropyrimidine dehydrogenase variants in toxicity to chemotherapy. Eur J Cancer. 2018;102:31–39. - PubMed
-
- EMA EMA recommendations on DPD testing prior to treatment with fluorouracil, capecitabine, tegafur and flucytosine. 2020. https://www.ema.europa.eu/en/news/ema-recommendations-dpd-testing-prior-... Available at: Accessed November 18, 2020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

