The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys
- PMID: 33895780
- PMCID: PMC8065334
- DOI: 10.1038/s41392-021-00591-7
The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys
Abstract
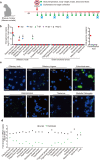
Neurological manifestations are frequently reported in the COVID-19 patients. Neuromechanism of SARS-CoV-2 remains to be elucidated. In this study, we explored the mechanisms of SARS-CoV-2 neurotropism via our established non-human primate model of COVID-19. In rhesus monkey, SARS-CoV-2 invades the CNS primarily via the olfactory bulb. Thereafter, viruses rapidly spread to functional areas of the central nervous system, such as hippocampus, thalamus, and medulla oblongata. The infection of SARS-CoV-2 induces the inflammation possibly by targeting neurons, microglia, and astrocytes in the CNS. Consistently, SARS-CoV-2 infects neuro-derived SK-N-SH, glial-derived U251, and brain microvascular endothelial cells in vitro. To our knowledge, this is the first experimental evidence of SARS-CoV-2 neuroinvasion in the NHP model, which provides important insights into the CNS-related pathogenesis of SARS-CoV-2.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

