Defining a minimal clinically meaningful difference in 12-month estimated glomerular filtration rate for clinical trials in deceased donor kidney transplantation
- PMID: 33896052
- PMCID: PMC8365649
- DOI: 10.1111/ctr.14326
Defining a minimal clinically meaningful difference in 12-month estimated glomerular filtration rate for clinical trials in deceased donor kidney transplantation
Abstract
Background: A Minimal Clinically Meaningful Difference (MCMD) has not been defined for Estimated glomerular filtration rate (eGFR). Our goal was to define the MCMD for eGFR anchored to kidney graft failure.
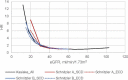
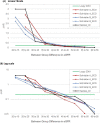
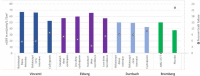
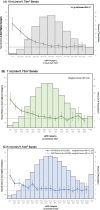
Methods: A systematic review of studies with 12-month eGFR and subsequent renal graft failure was conducted. For observational studies, we calculated hazard ratio (HR) differences between adjacent eGFR intervals weighted by population distribution. Interventional trials yielded therapeutically induced changes in eGFR and failure risk. OPTN data analysis divided 12-month eGFR into bands for Cox regressions comparing adjacent eGFR bands with a death-censored graft survival outcome.
Results: Observational studies indicated that lower eGFR was associated with increased death-censored graft failure risk; each 5 ml/min/1.73 m2 12-month eGFR band associated with a weighted incremental HR = 1.12 to 1.23. Clinical trial data found a 5 ml/min/1.73 m2 difference was associated with incremental HR = 1.16 to 1.35. OPTN analyses showed weighted mean HRs across 10, 7, and 5 ml/min/1.73 m2 bands of 1.47, 1.30, and 1.19.
Conclusions: A 5 ml/min/1.73 m2 difference in 12-month eGFR was consistently associated with ~20% increase in death-censored graft failure risk. The magnitude of effect has been interpreted as clinically meaningful in other disease states and should be considered the MCMD in renal transplantation clinical trials.
Keywords: clinical trial design; glomerular filtration rate; graft survival; kidney (allograft).
© 2021 The Authors. Clinical Transplantation published by John Wiley & Sons Ltd.
Conflict of interest statement
Sumit Mohan, Jesse Schold, and Matthew Weir have received research funding and/or consultancy fees unrelated to this study from Angion Biomedica. Tracy Mayne was an employee of Angion Biomedica at the time of this study. Robert Nordyke is an employee of Angion Biomedica and owns stock/stock options.
Figures
References
-
- Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713‐735. - PubMed
-
- International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM). Published March 31, 2020. Accessed May 29, 2020. https://www.cdc.gov/nchs/icd/icd10cm.htm
-
- Levin A, Stevens PE, Bilous RW, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1‐150. - PubMed
-
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407‐415. - PubMed
-
- Food Drug Administration Center for Drugs Evaluation Research . Guidance for industry: delayed graft function in kidney transplantation: developing drugs for prevention. 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

