Protective effect of berberine against LPS-induced endothelial cell injury via the JNK signaling pathway and autophagic mechanisms
- PMID: 33896366
- PMCID: PMC8806223
- DOI: 10.1080/21655979.2021.1915671
Protective effect of berberine against LPS-induced endothelial cell injury via the JNK signaling pathway and autophagic mechanisms
Abstract
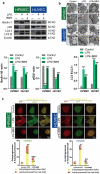
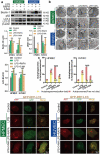
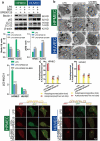
The role of autophagic mechanisms in the protective effect of berberine (BBR) on lipopolysaccharide (LPS)-induced injury in the endothelial cells human umbilical vein endothelial cells (HUVECs) and human pulmonary microvascular endothelial cells (HPMECs) was investigated. Cell viability, proliferation, and apoptosis were detected by the CCK-8 assay, the EdU kit, and flow cytometry, respectively, and autophagy-related protein expression, the number of autophagic vacuoles, and LC3 double-fluorescence were examined using western blot analysis, transmission electron microscopy, and confocal microscopy, respectively. LPS resulted in a decrease in the cell viability and proliferation of HUVECs and HPMECs and an increase in the number of apoptotic cells, while BBR treatment resulted in an increase in cell viability and proliferation, as well as a decrease in cell apoptosis. Furthermore, BBR could inhibit LPS-induced autophagy, as demonstrated by its inhibitory effects on the LC3-II/LC3-I ratio and Beclin-1 levels and its promotive effect on p62 expression. Addition of the autophagy inducer rapamycin (RAPA) aggravated LPS-induced injury, while treatment with the autophagy blocker 3-methyladenine (3-MA) attenuated the injury. Further, the protective effect of BBR was inhibited by rapamycin. JNK inhibition by SP600125 inhibited LPS-induced autophagy, and BBR could not alter the LPS-induced autophagy in HUVECs and HPMECs that were pretreated with SP600125. The present data indicate that BBR attenuated LPS-induced cell apoptosis by blocking JNK-mediated autophagy in HUVECs and HPMECs. Therefore, the JNK-mediated autophagy pathway could be a potential target for the prevention and treatment of cardiovascular disease.
Keywords: Berberine; JNK inhibitor; LPS; autophagy; endothelial cells.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
-
- Ebel B, Lemetais G, Beney L, et al. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr. 2014;54(2):175–189. - PubMed
-
- Wens I, Dalgas U, Stenager E, et al. Risk factors related to cardiovascular diseases and the metabolic syndrome in multiple sclerosis - a systematic review. Mult Scler. 2013;19(12):1556–1564. - PubMed
-
- Timmis A, Townsend N, Gale C, et al. European society of cardiology: cardiovascular disease statistics 2017. Eur Heart J. 2018;39(7):508–579. - PubMed
-
- Jensen HA, Mehta JL.. Endothelial cell dysfunction as a novel therapeutic target in atherosclerosis. Expert Rev Cardiovasc Ther. 2016;14(9):1021–1033. - PubMed
-
- Buttari B, Profumo E, Businaro R, et al. Oxidized haemoglobin-driven endothelial dysfunction and immune cell activation: novel therapeutic targets for atherosclerosis. Curr Med Chem. 2013;20(37):4806–4814. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
