Successful outcome after a single endoscopic fecal microbiota transplantation in a Shiba dog with non-responsive enteropathy during the treatment with chlorambucil
- PMID: 33896875
- PMCID: PMC8267193
- DOI: 10.1292/jvms.21-0063
Successful outcome after a single endoscopic fecal microbiota transplantation in a Shiba dog with non-responsive enteropathy during the treatment with chlorambucil
Abstract
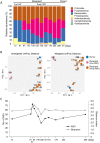
A 7-year 6-month-old, castrated male Shiba dog presented with a 1-month history of lethargy, anorexia, vomiting, and frequent watery diarrhea. Weight loss, hypoalbuminemia, anemia, and leukocytosis were detected at the first visit. The dog was diagnosed with non-responsive enteropathy (NRE) based on clinical and histopathological examinations. Since the dog did not respond to the immunosuppressive drugs, fecal microbiota transplantation (FMT) was performed during the treatment with chlorambucil. A single endoscopic FMT into the cecum and colon drastically recovered clinical signs and clinicopathological abnormalities and corrected dysbiosis in the dog. No recurrence or adverse events were observed. The present case report suggests that FMT, possibly together with chlorambucil, might be a treatment option for NRE in Shiba dogs that have poorer prognosis compared with other dog breeds.
Keywords: Shiba dog; dysbiosis; fecal microbiota transplantation; hypoalbuminemia; non-responsive enteropathy.
Conflict of interest statement
Ayaka Shima and Genki Ishihara are employees of Anicom Specialty Medical Institute Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Effect of faecal microbial transplantation on clinical outcome, faecal microbiota and metabolome in dogs with chronic enteropathy refractory to diet.Sci Rep. 2025 Apr 8;15(1):11957. doi: 10.1038/s41598-025-96906-7. Sci Rep. 2025. PMID: 40199985 Free PMC article.
-
Fecal Microbiota Transplantation in Dogs.Vet Clin North Am Small Anim Pract. 2021 Jan;51(1):219-233. doi: 10.1016/j.cvsm.2020.09.012. Epub 2020 Oct 29. Vet Clin North Am Small Anim Pract. 2021. PMID: 33131919 Review.
-
Effects of fecal microbiota transplantation on clinical outcomes and fecal microbiota of foals with diarrhea.J Vet Intern Med. 2024 Sep-Oct;38(5):2718-2728. doi: 10.1111/jvim.17185. Epub 2024 Sep 12. J Vet Intern Med. 2024. PMID: 39266472 Free PMC article.
-
Oral faecal microbiota transplantation for the treatment of Clostridium difficile-associated diarrhoea in a dog: a case report.BMC Vet Res. 2019 Jan 7;15(1):11. doi: 10.1186/s12917-018-1754-z. BMC Vet Res. 2019. PMID: 30616615 Free PMC article.
-
Role of the gastrointestinal microbiota in small animal health and disease.Vet Rec. 2017 Oct 7;181(14):370. doi: 10.1136/vr.103826. Epub 2017 Sep 15. Vet Rec. 2017. PMID: 28916525 Review.
Cited by
-
Companion Animal Model in Translational Oncology; Feline Oral Squamous Cell Carcinoma and Canine Oral Melanoma.Biology (Basel). 2021 Dec 31;11(1):54. doi: 10.3390/biology11010054. Biology (Basel). 2021. PMID: 35053051 Free PMC article. Review.
-
Fecal Microbiota Transplantation as a Treatment for Granulomatous Colitis in a French Bulldog: A Case Report.Microorganisms. 2025 Feb 8;13(2):366. doi: 10.3390/microorganisms13020366. Microorganisms. 2025. PMID: 40005733 Free PMC article.
-
Effect of faecal microbial transplantation on clinical outcome, faecal microbiota and metabolome in dogs with chronic enteropathy refractory to diet.Sci Rep. 2025 Apr 8;15(1):11957. doi: 10.1038/s41598-025-96906-7. Sci Rep. 2025. PMID: 40199985 Free PMC article.
-
Machine Learning and Canine Chronic Enteropathies: A New Approach to Investigate FMT Effects.Vet Sci. 2022 Sep 13;9(9):502. doi: 10.3390/vetsci9090502. Vet Sci. 2022. PMID: 36136718 Free PMC article.
-
Single Enema Fecal Microbiota Transplantation in Cats With Chronic Enteropathy.J Vet Intern Med. 2025 May-Jun;39(3):e70054. doi: 10.1111/jvim.70054. J Vet Intern Med. 2025. PMID: 40207935 Free PMC article.
References
-
- Bottero E., Benvenuti E., Ruggiero P.2017. Faecal microbiota transplantation in 16 dogs with idiopathic inflammatory bowel disease. Veterinaria 31: 1–12.

