Dietary Exposure to Low Levels of Crude Oil Affects Physiological and Morphological Phenotype in Adults and Their Eggs and Hatchlings of the King Quail (Coturnix chinensis)
- PMID: 33897469
- PMCID: PMC8063051
- DOI: 10.3389/fphys.2021.661943
Dietary Exposure to Low Levels of Crude Oil Affects Physiological and Morphological Phenotype in Adults and Their Eggs and Hatchlings of the King Quail (Coturnix chinensis)
Abstract
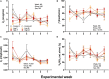
Despite the current knowledge of the devastating effects of external exposure to crude oil on animal mortality, the study of developmental, transgenerational effects of such exposure has received little attention. We used the king quail as an animal model to determine if chronic dietary exposure to crude oil in a parental population would affect morpho-physiological phenotypic variables in their immediate offspring generation. Adult quail were separated into three groups: (1) Control, and two experimental groups dietarily exposed for at least 3 weeks to (2) Low (800 PAH ng/g food), or (3) High (2,400 PAH ng/g food) levels of crude oil. To determine the parental influence on their offspring, we measured metabolic and respiratory physiology in exposed parents and in their non-exposed eggs and hatchlings. Body mass and numerous metabolic (e.g., O2 consumption, CO2 production) and respiratory (e.g., ventilation frequency and volume) variables did not vary between control and oil exposed parental groups. In contrast, blood PO2, PCO2, and SO2 varied among parental groups. Notably, water loss though the eggshell was increased in eggs from High oil level exposed parents. Respiratory variables of hatchlings did not vary between populations, but hatchlings obtained from High oil-exposed parents exhibited lower capacities to maintain body temperature while exposed to a cooling protocol in comparison to hatchlings from Low- and Control-derived parents. The present study demonstrates that parental exposure to crude oil via diet impacts some aspects of physiological performance of the subsequent first (F 1 ) generation.
Keywords: Transgenerational Inheritance; bird; crude oil; development; epigenetics; oxygen consumption; parental effects; physiology.
Copyright © 2021 Bautista, do Amaral-Silva, Dzialowski and Burggren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Albers P. H. (1977). “Effects of external applications of fuel oil on hatchability of mallard eggs,” in Fate and Effects of Petroleum Hydrocarbons in Marine Ecosystems and Organisms, Wolfe D. A., Anderson J. W. (Amsterdam: Elsevier; )158–163. 10.1016/b978-0-08-021613-3.50020-6 - DOI
-
- Alexander C. R., Hooper M. J., Cacela D., Smelker K. D., Calvin C. S., Dean K. M., et al. (2017). Reprint of: CYP1A protein expression and catalytic activity in double-crested cormorants experimentally exposed to deepwater horizon mississippi canyon 252 oil. Ecotoxicol. Environ. Safety 146 68–75. 10.1016/j.ecoenv.2017.05.015 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

