Evaluating the Velocity and Extent of Cortical Venous Filling in Patients With Severe Middle Cerebral Artery Stenosis or Occlusion
- PMID: 33897584
- PMCID: PMC8060485
- DOI: 10.3389/fneur.2021.610658
Evaluating the Velocity and Extent of Cortical Venous Filling in Patients With Severe Middle Cerebral Artery Stenosis or Occlusion
Abstract
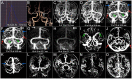
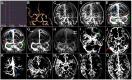
Objective: To investigate the velocity and extent of cortical venous filling (CVF) and its association with clinical manifestations in patients with severe stenosis or occlusion of the middle cerebral artery (MCA) using dynamic computed tomography angiography (CTA). Methods: Fifty-eight patients (36 symptomatic and 22 asymptomatic) with severe unilateral stenosis (≥70%) or occlusion of the MCA M1 segment who underwent dynamic CTA were included. Collateral status, antegrade flow, and CVF of each patient were observed using dynamic CTA. Three types of cortical veins were selected to observe the extent of CVF, and the absence of CVF (CVF-) was recorded. Based on the appearance of CVF in the superior sagittal sinus, instances of CVF, including early (CVF1), peak (CVF2), and late (CVF3) venous phases, were recorded. The differences in CVF times between the affected and contralateral hemispheres were represented as rCVFs, and CVF velocity was defined compared to the median time of each rCVF. Results: All CVF times in the affected hemisphere were longer than those in the contralateral hemisphere (p < 0.05). Patients with symptomatic MCA stenosis had more ipsilateral CVF- (p = 0.02) and more delayed CVF at rCVF2 and rCVF21 (rCVF2-rCVF1) (p = 0.03 and 0.001, respectively) compared to those with asymptomatic MCA stenosis. For symptomatic patients, fast CVF at rCVF21 was associated with poor collateral status (odds ratio [OR] 6.42, 95% confidence interval [CI] 1.37-30.05, p = 0.02), and ipsilateral CVF- in two cortical veins was associated with poor 3-month outcomes (adjusted OR 0.025, 95% CI 0.002-0.33, p = 0.005). Conclusions: Complete and fast CVF is essential for patients with symptomatic MCA stenosis or occlusion. The clinical value of additional CVF assessment should be explored in future studies to identify patients with severe MCA stenosis or occlusion at a higher risk of stroke occurrence and poor recovery.
Keywords: cortical venous filling; dynamic computed tomography angiography; middle cerebral artery; occlusion; severe stenosis.
Copyright © 2021 Lin, Cheng, Shi, Cai and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Evaluation of extent vs velocity of cortical venous filing in stroke outcome after endovascular thrombectomy.Neuroradiology. 2023 Jul;65(7):1155-1163. doi: 10.1007/s00234-023-03146-5. Epub 2023 Apr 6. Neuroradiology. 2023. PMID: 37022485
-
Cortical Venous Filling on Dynamic Computed Tomographic Angiography: A Novel Predictor of Clinical Outcome in Patients With Acute Middle Cerebral Artery Stroke.Stroke. 2016 Mar;47(3):762-7. doi: 10.1161/STROKEAHA.115.012279. Epub 2016 Jan 26. Stroke. 2016. PMID: 26814234 Clinical Trial.
-
Assessment of Collateral Status by Dynamic CT Angiography in Acute MCA Stroke: Timing of Acquisition and Relationship with Final Infarct Volume.AJNR Am J Neuroradiol. 2016 Jul;37(7):1231-6. doi: 10.3174/ajnr.A4746. Epub 2016 Mar 31. AJNR Am J Neuroradiol. 2016. PMID: 27032971 Free PMC article.
-
Delay of late-venous phase cortical vein filling in acute ischemic stroke patients: Associations with collateral status.J Cereb Blood Flow Metab. 2017 Feb;37(2):671-682. doi: 10.1177/0271678X16637611. Epub 2016 Jul 20. J Cereb Blood Flow Metab. 2017. PMID: 26965242 Free PMC article.
-
Internal Carotid Artery Stenosis and Collateral Recruitment in Stroke Patients.Clin Neuroradiol. 2018 Sep;28(3):339-344. doi: 10.1007/s00062-017-0568-x. Epub 2017 Apr 24. Clin Neuroradiol. 2018. PMID: 28439614 Free PMC article.
Cited by
-
Unfavorable venous outflow correlates with poor prognosis in acute ischemic stroke due to large vessel occlusion (AIS-LVO) patients assessed dynamically and quantitatively based on four-dimensional computed tomography angiography/perfusion (4D-CTA/CTP).Quant Imaging Med Surg. 2025 Apr 1;15(4):2865-2880. doi: 10.21037/qims-24-669. Epub 2025 Mar 28. Quant Imaging Med Surg. 2025. PMID: 40235806 Free PMC article.
-
Overlooked cerebral venous features associated with disease progression in moyamoya disease: insights from a single-center case-control study.Neurosurg Rev. 2025 Feb 17;48(1):246. doi: 10.1007/s10143-025-03401-8. Neurosurg Rev. 2025. PMID: 39961880
-
Evaluation of extent vs velocity of cortical venous filing in stroke outcome after endovascular thrombectomy.Neuroradiology. 2023 Jul;65(7):1155-1163. doi: 10.1007/s00234-023-03146-5. Epub 2023 Apr 6. Neuroradiology. 2023. PMID: 37022485
-
Venous collaterals in acute ischemic stroke patients after endovascular treatments: a novel scoring system using 4D computed tomography angiography.Quant Imaging Med Surg. 2022 Nov;12(11):5030-5043. doi: 10.21037/qims-22-245. Quant Imaging Med Surg. 2022. PMID: 36330192 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

