The Regulation of Adaptation to Cold and Drought Stresses in Poa crymophila Keng Revealed by Integrative Transcriptomics and Metabolomics Analysis
- PMID: 33897721
- PMCID: PMC8058472
- DOI: 10.3389/fpls.2021.631117
The Regulation of Adaptation to Cold and Drought Stresses in Poa crymophila Keng Revealed by Integrative Transcriptomics and Metabolomics Analysis
Abstract
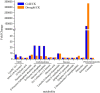
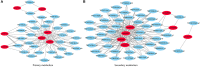
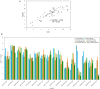
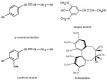
Poa crymophila Keng is highly adaptable to long-term low temperature and drought conditions, making it a desirable foraging grass of the Qinghai-Tibet Plateau. Here, the widely targeted metabolomics and comparative transcriptome analyses were utilized for the discovery of metabolites and genes in P. crymophila in response to cold and drought stresses. P. crymophila were exposed to -5°C for 24 h and recovered to 22°C for 48 h, as well as drought for 10 days followed by re-watering for 1 day. In total, 779 metabolic features were assigned to metabolites and 167,845 unigenes were generated. Seventeen compounds showed significant up-regulation (variable importance in project >1) under both stresses in the metabolic profiling, mainly annotated as carbohydrates, flavones, and phenylpropanoids. The genes which were positively correlated with these metabolites were assigned to pathways (sucrose-starch, raffinose, phenylpropanoid, and flavone metabolism) using the Mapman software package. Alpha-amylase, beta-fructofuranosidase, and sugar transport genes degraded the glucose and starch to small molecule sugars for the purpose of osmotic adjustment and to provide more energy for the growth of P. crymophila in an adverse environment. The induction of cinnamoyl-CoA reductase (CCR) and the MYB gene as well as the sharp increase in schizandrin, a kind of lignan, showed that this likely has the closest connection with the tolerance to both stresses. Four significantly induced flavone compounds are probably involved in reducing oxidative damage. Our results indicated that activation of the phenlypropanoid pathway plays the primary role in P. crymophila adapting to harsh environments. This study showed the mechanism of P. crymophila responding to both cold and drought stresses and showed the discovery of a new biological regulator against stresses.
Keywords: Poa crymophila Keng; carbohydrates; cold and drought; flavone; phenylpropanoids; transcriptome; widely targeted metabolomics.
Copyright © 2021 Wang, Li, Li, He, Hou and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Flexible response and rapid recovery strategies of the plateau forage Poa crymophila to cold and drought.Front Plant Sci. 2022 Nov 8;13:970496. doi: 10.3389/fpls.2022.970496. eCollection 2022. Front Plant Sci. 2022. PMID: 36426156 Free PMC article.
-
Tissue-specific transcriptomic analysis reveals the molecular mechanisms responsive to cold stress in Poa crymophila, and development of EST-SSR markers linked to cold tolerance candidate genes.BMC Plant Biol. 2025 Mar 19;25(1):360. doi: 10.1186/s12870-025-06383-3. BMC Plant Biol. 2025. PMID: 40102740 Free PMC article.
-
Transcriptome and metabolite analysis reveal the drought tolerance of foxtail millet significantly correlated with phenylpropanoids-related pathways during germination process under PEG stress.BMC Plant Biol. 2020 Jun 15;20(1):274. doi: 10.1186/s12870-020-02483-4. BMC Plant Biol. 2020. PMID: 32539796 Free PMC article.
-
Sugar metabolism in the desiccation tolerant grass Oropetium thomaeum in response to environmental stresses.Plant Sci. 2018 May;270:30-36. doi: 10.1016/j.plantsci.2018.02.004. Epub 2018 Feb 8. Plant Sci. 2018. PMID: 29576083
-
Gene regulation network behind drought escape, avoidance and tolerance strategies in black poplar (Populus nigra L.).Plant Physiol Biochem. 2017 Jun;115:183-199. doi: 10.1016/j.plaphy.2017.03.020. Epub 2017 Mar 28. Plant Physiol Biochem. 2017. PMID: 28376411
Cited by
-
Changes of crystalline structure and physicochemical properties of Pueraria lobata var. thomsonii starch under water deficit.PLoS One. 2024 Jul 3;19(7):e0304373. doi: 10.1371/journal.pone.0304373. eCollection 2024. PLoS One. 2024. PMID: 38959223 Free PMC article.
-
Flexible response and rapid recovery strategies of the plateau forage Poa crymophila to cold and drought.Front Plant Sci. 2022 Nov 8;13:970496. doi: 10.3389/fpls.2022.970496. eCollection 2022. Front Plant Sci. 2022. PMID: 36426156 Free PMC article.
-
Integrative Analysis of Elicitor-Induced Camptothecin Biosynthesis in Camptotheca acuminata Plantlets Through a Combined Omics Approach.Front Plant Sci. 2022 Mar 24;13:851077. doi: 10.3389/fpls.2022.851077. eCollection 2022. Front Plant Sci. 2022. PMID: 35401649 Free PMC article.
-
Comparative Transcriptome Analysis Reveals the Genes and Pathways Related to Wheat Root Hair Length.Int J Mol Sci. 2024 Feb 8;25(4):2069. doi: 10.3390/ijms25042069. Int J Mol Sci. 2024. PMID: 38396749 Free PMC article.
-
Climate-Affected Australian Tropical Montane Cloud Forest Plants: Metabolomic Profiles, Isolated Phytochemicals, and Bioactivities.Plants (Basel). 2024 Apr 3;13(7):1024. doi: 10.3390/plants13071024. Plants (Basel). 2024. PMID: 38611553 Free PMC article. Review.
References
-
- Amelia V. D., Aversano R., Chiaiese P., Carputo D. (2018). The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding. Phytochem. Rev. 17, 611–625. 10.1007/s11101-018-9568-y - DOI
-
- Andrade E. R., Ribeiro V. N., Azevedo C. V. G., Chiorato A. F., Williams T. C. R., Carbonell S. A. M. (2016). Biochemical indicators of drought tolerance in the common bean (Phaseolus vulgaris L.). Euphytica 210, 277–289. 10.1007/s10681-016-1720-4 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

