Changes in Leaf-Level Nitrogen Partitioning and Mesophyll Conductance Deliver Increased Photosynthesis for Lolium perenne Leaves Engineered to Accumulate Lipid Carbon Sinks
- PMID: 33897730
- PMCID: PMC8063613
- DOI: 10.3389/fpls.2021.641822
Changes in Leaf-Level Nitrogen Partitioning and Mesophyll Conductance Deliver Increased Photosynthesis for Lolium perenne Leaves Engineered to Accumulate Lipid Carbon Sinks
Abstract
Diacylglycerol acyl-transferase (DGAT) and cysteine oleosin (CO) expression confers a novel carbon sink (of encapsulated lipid droplets) in leaves of Lolium perenne and has been shown to increase photosynthesis and biomass. However, the physiological mechanism by which DGAT + CO increases photosynthesis remains unresolved. To evaluate the relationship between sink strength and photosynthesis, we examined fatty acids (FA), water-soluble carbohydrates (WSC), gas exchange parameters and leaf nitrogen for multiple DGAT + CO lines varying in transgene accumulation. To identify the physiological traits which deliver increased photosynthesis, we assessed two important determinants of photosynthetic efficiency, CO2 conductance from atmosphere to chloroplast, and nitrogen partitioning between different photosynthetic and non-photosynthetic pools. We found that DGAT + CO accumulation increased FA at the expense of WSC in leaves of L. perenne and for those lines with a significant reduction in WSC, we also observed an increase in photosynthesis and photosynthetic nitrogen use efficiency. DGAT + CO L. perenne displayed no change in rubisco content or Vcmax but did exhibit a significant increase in specific leaf area (SLA), stomatal and mesophyll conductance, and leaf nitrogen allocated to photosynthetic electron transport. Collectively, we showed that increased carbon demand via DGAT+CO lipid sink accumulation can induce leaf-level changes in L. perenne which deliver increased rates of photosynthesis and growth. Carbon sinks engineered within photosynthetic cells provide a promising new strategy for increasing photosynthesis and crop productivity.
Keywords: Lolium perenne; cysteine oleosin; diacylglycerol acyl-transferase; lipid; photosynthesis; sink strength.
Copyright © 2021 Cooney, Beechey-Gradwell, Winichayakul, Richardson, Crowther, Anderson, Scott, Bryan and Roberts.
Conflict of interest statement
The DGAT+CO ryegrass material examined in this study was generated with funding from DairyNZ, PGG Wrightson Seeds and Grasslanz Technology. The research conducted here, including all experimental designs and analyses was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
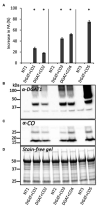

 ) and low molecular weight carbohydrates (shaded white,
) and low molecular weight carbohydrates (shaded white,  ) in the leaves of five DGAT + CO transformed L. perenne lines and respective non-transformed controls. Matching genetic backgrounds are grouped together. n = 10. **Statistically differs from NT, p < 0.01.
) in the leaves of five DGAT + CO transformed L. perenne lines and respective non-transformed controls. Matching genetic backgrounds are grouped together. n = 10. **Statistically differs from NT, p < 0.01.
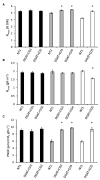
 ), NT2 and DGAT + CO3-4 (
), NT2 and DGAT + CO3-4 ( ) and NT3 and DGAT + CO5 (
) and NT3 and DGAT + CO5 ( ). Trendline represents NT2 and NT3 derived lines. Photosynthesis measured at 600 μmol photons m-2 s-1.
). Trendline represents NT2 and NT3 derived lines. Photosynthesis measured at 600 μmol photons m-2 s-1.
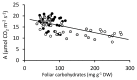
 ) and NT control (NT3; closed circles
) and NT control (NT3; closed circles  ) grown under 1-7 mM NO3- supply. Means ± SE; n = 3 for 1, 2, and 3 mM treated plants, n = 5 for 5 and 7.5 mM treated plants.
) grown under 1-7 mM NO3- supply. Means ± SE; n = 3 for 1, 2, and 3 mM treated plants, n = 5 for 5 and 7.5 mM treated plants.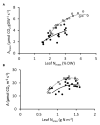
 ) and NT control NT3 (closed circles;
) and NT control NT3 (closed circles;  ) grown under 1–7.5 mM NO3− supply. Photosynthesis measurements were made at 1500 μmol photons m−2 s−1.
) grown under 1–7.5 mM NO3− supply. Photosynthesis measurements were made at 1500 μmol photons m−2 s−1.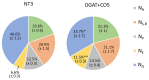
Similar articles
-
Storing carbon in leaf lipid sinks enhances perennial ryegrass carbon capture especially under high N and elevated CO2.J Exp Bot. 2020 Apr 6;71(7):2351-2361. doi: 10.1093/jxb/erz494. J Exp Bot. 2020. PMID: 31679036 Free PMC article.
-
Interspecific differences in how sink-source imbalance causes photosynthetic downregulation among three legume species.Ann Bot. 2019 Mar 14;123(4):715-726. doi: 10.1093/aob/mcy204. Ann Bot. 2019. PMID: 30517608 Free PMC article.
-
Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field.J Exp Bot. 2009;60(8):2249-70. doi: 10.1093/jxb/erp036. Epub 2009 Apr 23. J Exp Bot. 2009. PMID: 19395391 Review.
-
Within-branch photosynthetic gradients are more related to the coordinated investments of nitrogen and water than leaf mass per area.Plant Physiol Biochem. 2023 May;198:107681. doi: 10.1016/j.plaphy.2023.107681. Epub 2023 Apr 6. Plant Physiol Biochem. 2023. PMID: 37054614
-
Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts.J Plant Physiol. 2016 Sep 20;203:84-94. doi: 10.1016/j.jplph.2016.04.002. Epub 2016 Apr 7. J Plant Physiol. 2016. PMID: 27083537 Review.
Cited by
-
Epichloë fungal endophyte interactions in perennial ryegrass (Lolium perenne L.) modified to accumulate foliar lipids for increased energy density.BMC Plant Biol. 2023 Dec 11;23(1):636. doi: 10.1186/s12870-023-04635-8. BMC Plant Biol. 2023. PMID: 38072924 Free PMC article.
-
Toward sustainable crops: integrating vegetative (non-seed) lipid storage, carbon-nitrogen dynamics, and redox regulation.Front Plant Sci. 2025 Jun 3;16:1589127. doi: 10.3389/fpls.2025.1589127. eCollection 2025. Front Plant Sci. 2025. PMID: 40530288 Free PMC article. Review.
-
An alternative angiosperm DGAT1 topology and potential motifs in the N-terminus.Front Plant Sci. 2022 Sep 16;13:951389. doi: 10.3389/fpls.2022.951389. eCollection 2022. Front Plant Sci. 2022. PMID: 36186081 Free PMC article.
-
Elucidation of Triacylglycerol Overproduction in the C4 Bioenergy Crop Sorghum bicolor by Constraint-Based Analysis.Front Plant Sci. 2022 Feb 17;13:787265. doi: 10.3389/fpls.2022.787265. eCollection 2022. Front Plant Sci. 2022. PMID: 35251073 Free PMC article.
-
Callus Induction from Diverse Explants and Genotypes Enables Robust Transformation of Perennial Ryegrass (Lolium perenne L.).Plants (Basel). 2022 Aug 5;11(15):2054. doi: 10.3390/plants11152054. Plants (Basel). 2022. PMID: 35956532 Free PMC article.
References
-
- Ainsworth E. A., Rogers A., Nelson R., Long S. P. (2004). Testing the “source–sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric. For. Meteorol. 122, 85–94. 10.1016/j.agrformet.2003.09.002 - DOI
-
- Altpeter F., Xu J., Ahmed S. (2000). Generation of large numbers of independently transformed fertile perennial ryegrass (Lolium perenne L.) plants of forage- and turf-type cultivars. Mol. Breed. 6, 519–528. 10.1023/A:1026589804034 - DOI
-
- Andrews M., Love B. G., Sprent J. J. (1989). The effects of different external nitrate concentrations on growth of Phaseolus vulgaris cv. Seafarer at chilling temperatures. Ann. Appl. Biol. 114, 195–204. 10.1111/j.1744-7348.1989.tb06800.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

