Expression of a Fungal Lectin in Arabidopsis Enhances Plant Growth and Resistance Toward Microbial Pathogens and a Plant-Parasitic Nematode
- PMID: 33897746
- PMCID: PMC8063123
- DOI: 10.3389/fpls.2021.657451
Expression of a Fungal Lectin in Arabidopsis Enhances Plant Growth and Resistance Toward Microbial Pathogens and a Plant-Parasitic Nematode
Abstract
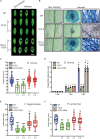
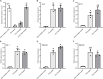
Coprinopsis cinerea lectin 2 (CCL2) is a fucoside-binding lectin from the basidiomycete C. cinerea that is toxic to the bacterivorous nematode Caenorhabditis elegans as well as animal-parasitic and fungivorous nematodes. We expressed CCL2 in Arabidopsis to assess its protective potential toward plant-parasitic nematodes. Our results demonstrate that expression of CCL2 enhances host resistance against the cyst nematode Heterodera schachtii. Surprisingly, CCL2-expressing plants were also more resistant to fungal pathogens including Botrytis cinerea, and the phytopathogenic bacterium Pseudomonas syringae. In addition, CCL2 expression positively affected plant growth indicating that CCL2 has the potential to improve two important agricultural parameters namely biomass production and general disease resistance. The mechanism of the CCL2-mediated enhancement of plant disease resistance depended on fucoside-binding by CCL2 as transgenic plants expressing a mutant version of CCL2 (Y92A), compromised in fucoside-binding, exhibited wild type (WT) disease susceptibility. The protective effect of CCL2 did not seem to be direct as the lectin showed no growth-inhibition toward B. cinerea in in vitro assays. We detected, however, a significantly enhanced transcriptional induction of plant defense genes in CCL2- but not CCL2-Y92A-expressing lines in response to infection with B. cinerea compared to WT plants. This study demonstrates a potential of fungal defense lectins in plant protection beyond their use as toxins.
Keywords: Arabidopsis; Botrytis cinerea; Coprinopsis cinerea lectin 2; Heterodera schachtii; Pseudomonas syringae.
Copyright © 2021 Moradi, El-Shetehy, Gamir, Austerlitz, Dahlin, Wieczorek, Künzler and Mauch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Inhibition of Haemonchus contortus larval development by fungal lectins.Parasit Vectors. 2015 Aug 19;8:425. doi: 10.1186/s13071-015-1032-x. Parasit Vectors. 2015. PMID: 26283415 Free PMC article.
-
The role of AtPP2-A3 and AtPP2-A8 genes encoding Nictaba-related lectin domains in the defense response of Arabidopsis thaliana to Heterodera schachtii.Planta. 2023 Jul 8;258(2):40. doi: 10.1007/s00425-023-04196-y. Planta. 2023. PMID: 37420105 Free PMC article.
-
Marasmius oreades agglutinin enhances resistance of Arabidopsis against plant-parasitic nematodes and a herbivorous insect.BMC Plant Biol. 2021 Sep 1;21(1):402. doi: 10.1186/s12870-021-03186-0. BMC Plant Biol. 2021. PMID: 34470613 Free PMC article.
-
The BOS loci of Arabidopsis are required for resistance to Botrytis cinerea infection.Plant J. 2004 Nov;40(4):558-74. doi: 10.1111/j.1365-313X.2004.02232.x. Plant J. 2004. PMID: 15500471
-
Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi.RNA Biol. 2017 Apr 3;14(4):421-428. doi: 10.1080/15476286.2017.1291112. Epub 2017 Mar 7. RNA Biol. 2017. PMID: 28267415 Free PMC article. Review.
Cited by
-
TIR1/AFB proteins: Active players in abiotic and biotic stress signaling.Front Plant Sci. 2022 Nov 29;13:1083409. doi: 10.3389/fpls.2022.1083409. eCollection 2022. Front Plant Sci. 2022. PMID: 36523629 Free PMC article. Review.
-
Dual RNA-Seq Profiling Unveils Mycoparasitic Activities of Trichoderma atroviride against Haploid Armillaria ostoyae in Antagonistic Interaction Assays.Microbiol Spectr. 2023 Jun 15;11(3):e0462622. doi: 10.1128/spectrum.04626-22. Epub 2023 May 4. Microbiol Spectr. 2023. PMID: 37140425 Free PMC article.
-
Fungal β-Glucans Shape Innate Immune Responses in Human Peripheral Blood Mononuclear Cells (PBMCs): An In Vitro Study on PRR Regulation, Cytokine Expression, and Oxidative Balance.Int J Mol Sci. 2025 Jul 4;26(13):6458. doi: 10.3390/ijms26136458. Int J Mol Sci. 2025. PMID: 40650232 Free PMC article.
-
Sweet Modifications Modulate Plant Development.Biomolecules. 2021 May 18;11(5):756. doi: 10.3390/biom11050756. Biomolecules. 2021. PMID: 34070047 Free PMC article. Review.
-
BOL Lectin: A Protein Derived from Cauliflower Exhibits Antibiofilm Activity in In Vitro Assays Against Staphylococcus aureus.Microorganisms. 2025 Aug 15;13(8):1901. doi: 10.3390/microorganisms13081901. Microorganisms. 2025. PMID: 40871405 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

