G6PD Polymorphisms and Hemolysis After Antimalarial Treatment With Low Single-Dose Primaquine: A Pooled Analysis of Six African Clinical Trials
- PMID: 33897764
- PMCID: PMC8062977
- DOI: 10.3389/fgene.2021.645688
G6PD Polymorphisms and Hemolysis After Antimalarial Treatment With Low Single-Dose Primaquine: A Pooled Analysis of Six African Clinical Trials
Abstract
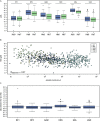
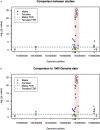
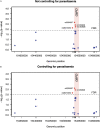
Primaquine (PQ) is an antimalarial drug with the potential to reduce malaria transmission due to its capacity to clear mature Plasmodium falciparum gametocytes in the human host. However, the large-scale roll-out of PQ has to be counterbalanced by the additional risk of drug-induced hemolysis in individuals suffering from Glucose-6-phospate dehydrogenase (G6PD) deficiency, a genetic condition determined by polymorphisms on the X-linked G6PD gene. Most studies on G6PD deficiency and PQ-associated hemolysis focused on the G6PD A- variant, a combination of the two single nucleotide changes G202A (rs1050828) and A376G (rs1050829), although other polymorphisms may play a role. In this study, we tested the association of 20 G6PD single nucleotide polymorphisms (SNPs) with hemolysis measured seven days after low single dose of PQ given at the dose of 0.1 mg/kg to 0.75 mg/kg in 957 individuals from 6 previously published clinical trials investigating the safety and efficacy of this drug spanning five African countries. After adjusting for inter-study effects, age, gender, baseline hemoglobin level, PQ dose, and parasitemia at screening, our analysis showed putative association signals from the common G6PD mutation, A376G [-log10(p-value) = 2.44] and two less-known SNPs, rs2230037 [-log10(p-value] = 2.60), and rs28470352 [-log10(p-value) = 2.15]; A376G and rs2230037 were in very strong linkage disequilibrium with each other (R 2 = 0.978). However, when the effects of these SNPs were included in the same regression model, the subsequent associations were in the borderline of statistical significance. In conclusion, whilst a role for the A- variant is well established, we did not observe an important additional role for other G6PD polymorphisms in determining post-treatment hemolysis in individuals treated with low single-dose PQ.
Keywords: clinical trials; drug safety; genetic association study; hemoglobin; malaria.
Copyright © 2021 Sepúlveda, Grignard, Curry, Mahey, Bastiaens, Tiono, Okebe, Coulibaly, Gonçalves, Affara, Ouédraogo, Bougouma, Sanou, Nébié, Lanke, Sirima, Dicko, d’Alessandro, Clark, Campino, Chen, Eziefula, Gosling, Bousema and Drakeley.
Conflict of interest statement
JC and LM were employed by the company LGC Genomics. The remaining authors declare that the research was conducted in the absence of any commercial and financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Awandu S. S., Raman J., Makhanthisa T. I., Kruger P., Frean J., Bousema T., et al. (2018). Understanding human genetic factors influencing primaquine safety and efficacy to guide primaquine roll-out in a pre-elimination setting in southern Africa. Malar. J. 17:120. 10.1186/s12936-018-2271-z - DOI - PMC - PubMed
-
- Bastiaens G. J. H., Tiono A. B., Okebe J., Pett H. E., Coulibaly S. A., Gonçalves B. P., et al. (2018). Safety of single low-dose primaquine in glucose-6-phosphate dehydrogenase deficient falciparum-infected African males: two open-label, randomized, safety trials. PLoS One 13:e0190272. 10.1371/journal.pone.0190272 - DOI - PMC - PubMed
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57 289–300.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

