Epstein-Barr Virus Infection in the Development of Neurological Disorders
- PMID: 33897799
- PMCID: PMC8059607
- DOI: 10.1016/j.ddmod.2020.01.001
Epstein-Barr Virus Infection in the Development of Neurological Disorders
Abstract
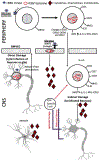
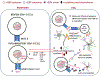
Epstein-Barr Virus (EBV) is a ubiquitous human herpesvirus that contributes to the etiology of diverse human cancers and auto-immune diseases. EBV establishes a relatively benign, long-term latent infection in over 90 percent of the adult population. Yet, it also increases risk for certain cancers and auto-immune disorders depending on complex viral, host, and environmental factors that are only partly understood. EBV latent infection is found predominantly in memory B-cells, but the natural infection cycle and pathological aberrations enable EBV to infect numerous other cell types, including oral, nasopharyngeal, and gastric epithelia, B-, T-, and NK-lymphoid cells, myocytes, adipocytes, astrocytes, and neurons. EBV infected cells, free virus, and gene products can also be found in the CNS. In addition to the direct effects of EBV on infected cells and tissue, the effect of chronic EBV infection on the immune system is also thought to contribute to pathogenesis, especially auto-immune disease. Here, we review properties of EBV infection that may shed light on its potential pathogenic role in neurological disorders.
Keywords: CNS; Epstein-barr virus; PCNLS; encephalitis; latent infection; lytic infection; multiple sclerosis.
Conflict of interest statement
Conflicts of Interest PML declares financial conflict of interest relationship with Cullinan Apollo, Inc. and ownership interest in Vironika, LLC.
Figures

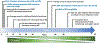
References
-
- Epstein A, Eastwood MA. 1995. Denis Parsons Burkitt. 28 February 1911–1993., p 90, Biographical memoirs of fellows of the Royal Society, vol 41. - PubMed
-
- Burkitt D. 1958. A sarcoma involving the jaws in African children. Br J Surg 46:218–23. - PubMed
-
- O’Conor GT, Davies JN. 1960. Malignant tumors in African children. With special reference to malignant lymphoma. J Pediatr 56:526–35. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
