True Nature of the Transition-Metal Carbide/Liquid Interface Determines Its Reactivity
- PMID: 33898080
- PMCID: PMC8057231
- DOI: 10.1021/acscatal.1c00415
True Nature of the Transition-Metal Carbide/Liquid Interface Determines Its Reactivity
Abstract
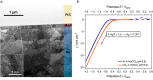
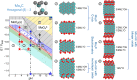
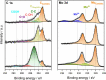
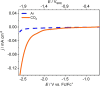
Compound materials, such as transition-metal (TM) carbides, are anticipated to be effective electrocatalysts for the carbon dioxide reduction reaction (CO2RR) to useful chemicals. This expectation is nurtured by density functional theory (DFT) predictions of a break of key adsorption energy scaling relations that limit CO2RR at parent TMs. Here, we evaluate these prospects for hexagonal Mo2C in aqueous electrolytes in a multimethod experiment and theory approach. We find that surface oxide formation completely suppresses the CO2 activation. The oxides are stable down to potentials as low as -1.9 V versus the standard hydrogen electrode, and solely the hydrogen evolution reaction (HER) is found to be active. This generally points to the absolute imperative of recognizing the true interface establishing under operando conditions in computational screening of catalyst materials. When protected from ambient air and used in nonaqueous electrolyte, Mo2C indeed shows CO2RR activity.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Zhao Z.-J.; Liu S.; Zha S.; Cheng D.; Studt F.; Henkelman G.; Gong J. Theory-Guided Design of Catalytic Materials Using Scaling Relationships and Reactivity Descriptors. Nat. Rev. Mater. 2019, 4, 792–804. 10.1038/s41578-019-0152-x. - DOI
-
- Védrine J. Heterogeneous Catalysis on Metal Oxides. Catalysts 2017, 7, 341 10.3390/catal7110341. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
