Chemically Engineered Immune Cell-Derived Microrobots and Biomimetic Nanoparticles: Emerging Biodiagnostic and Therapeutic Tools
- PMID: 33898169
- PMCID: PMC8061401
- DOI: 10.1002/advs.202002499
Chemically Engineered Immune Cell-Derived Microrobots and Biomimetic Nanoparticles: Emerging Biodiagnostic and Therapeutic Tools
Abstract
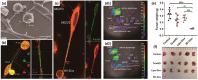
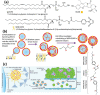
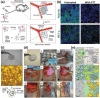
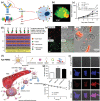
Over the past decades, considerable attention has been dedicated to the exploitation of diverse immune cells as therapeutic and/or diagnostic cell-based microrobots for hard-to-treat disorders. To date, a plethora of therapeutics based on alive immune cells, surface-engineered immune cells, immunocytes' cell membranes, leukocyte-derived extracellular vesicles or exosomes, and artificial immune cells have been investigated and a few have been introduced into the market. These systems take advantage of the unique characteristics and functions of immune cells, including their presence in circulating blood and various tissues, complex crosstalk properties, high affinity to different self and foreign markers, unique potential of their on-demand navigation and activity, production of a variety of chemokines/cytokines, as well as being cytotoxic in particular conditions. Here, the latest progress in the development of engineered therapeutics and diagnostics inspired by immune cells to ameliorate cancer, inflammatory conditions, autoimmune diseases, neurodegenerative disorders, cardiovascular complications, and infectious diseases is reviewed, and finally, the perspective for their clinical application is delineated.
Keywords: artificial dendritic cell and extracellular vesicle; biomimetic drug delivery; engineered immune cell; immune cell membrane; nanomedicine.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



























Similar articles
-
Membrane engineering of cell membrane biomimetic nanoparticles for nanoscale therapeutics.Clin Transl Med. 2021 Feb;11(2):e292. doi: 10.1002/ctm2.292. Clin Transl Med. 2021. PMID: 33635002 Free PMC article. Review.
-
The potential of biomimetic nanoparticles for tumor-targeted drug delivery.Nanomedicine (Lond). 2018 Aug;13(16):2099-2118. doi: 10.2217/nnm-2018-0017. Epub 2018 Sep 18. Nanomedicine (Lond). 2018. PMID: 30226404 Review.
-
Biomimicking Extracellular Vesicles with Fully Artificial Ones: A Rational Design of EV-BIOMIMETICS toward Effective Theranostic Tools in Nanomedicine.ACS Biomater Sci Eng. 2023 Nov 13;9(11):5924-5932. doi: 10.1021/acsbiomaterials.2c01025. Epub 2022 Dec 19. ACS Biomater Sci Eng. 2023. PMID: 36535896 Free PMC article. Review.
-
Recent Advances of Membrane-Cloaked Nanoplatforms for Biomedical Applications.Bioconjug Chem. 2018 Apr 18;29(4):838-851. doi: 10.1021/acs.bioconjchem.8b00103. Epub 2018 Mar 20. Bioconjug Chem. 2018. PMID: 29509403 Review.
-
Nanomedicines in oral cancer: inspiration comes from extracellular vesicles and biomimetic nanoparticles.Nanomedicine (Lond). 2022 Oct;17(23):1761-1778. doi: 10.2217/nnm-2022-0142. Epub 2023 Jan 17. Nanomedicine (Lond). 2022. PMID: 36647844 Review.
Cited by
-
CRISPR/Cas9 therapeutics: progress and prospects.Signal Transduct Target Ther. 2023 Jan 16;8(1):36. doi: 10.1038/s41392-023-01309-7. Signal Transduct Target Ther. 2023. PMID: 36646687 Free PMC article. Review.
-
Macrophage membrane-functionalized nanotherapeutics for tumor targeted therapy.Theranostics. 2025 Mar 31;15(10):4823-4847. doi: 10.7150/thno.108875. eCollection 2025. Theranostics. 2025. PMID: 40225567 Free PMC article. Review.
-
Abalone-Inspired Adhesive and Photo-Responsive Microparticle Delivery Systems for Periodontal Drug Therapy.Adv Sci (Weinh). 2022 Oct;9(30):e2202829. doi: 10.1002/advs.202202829. Epub 2022 Aug 30. Adv Sci (Weinh). 2022. PMID: 36041051 Free PMC article.
-
COVID-19 inflammation and implications in drug delivery.J Control Release. 2022 Jun;346:260-274. doi: 10.1016/j.jconrel.2022.04.027. Epub 2022 Apr 27. J Control Release. 2022. PMID: 35469984 Free PMC article. Review.
-
Suction-Cup-Inspired Adhesive Micromotors for Drug Delivery.Adv Sci (Weinh). 2022 Jan;9(1):e2103384. doi: 10.1002/advs.202103384. Epub 2021 Nov 1. Adv Sci (Weinh). 2022. PMID: 34726356 Free PMC article.
References
-
- a) Sheikhpour M., Barani L., Kasaeian A., J. Controlled Release 2017, 253, 97; - PubMed
- b) El‐Ashmawy N., El‐Zamarany E., Khedr E., El‐Bahrawy H., El‐Feky O., Clin. Transl. Oncol. 2019, 21, 636; - PubMed
- c) Zubizarreta I., Flórez‐Grau G., Vila G., Cabezón R., España C., Andorra M., Saiz A., Llufriu S., Sepulveda M., Sola‐Valls N., Proc. Natl. Acad. Sci. USA 2019, 116, 8463; - PMC - PubMed
- d) Kono Y., Gogatsubo S., Ohba T., Fujita T., Drug Delivery 2019, 26, 935. - PMC - PubMed
-
- a) Molinaro R., Martinez J. O., Zinger A., De Vita A., Storci G., Arrighetti N., De Rosa E., Hartman K. A., Basu N., Taghipour N., Biomater. Sci. 2020, 8, 333; - PubMed
- b) Gudi R. R., Karumuthil‐Melethil S., Perez N., Li G., Vasu C., Sci. Rep. 2019, 9, 12065; - PMC - PubMed
- c) Bhattacharyya S., Ghosh S. S., ACS Omega 2020, 5, 1572; - PMC - PubMed
- d) Zhang Q., Wu J., Wang J., Wang X., Wu C., Chen M., Wu Q., Lesniak M. S., Mi Y., Cheng Y., Angew. Chem. 2020, 132, 3761. - PubMed
-
- Ye Z.‐P.‐P., Ai X.‐L., Faramand A. M., Fang F., J. Nanosci. Nanotechnol. 2018, 18, 471. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
