Stretchable, Fully Polymeric Electrode Arrays for Peripheral Nerve Stimulation
- PMID: 33898185
- PMCID: PMC8061359
- DOI: 10.1002/advs.202004033
Stretchable, Fully Polymeric Electrode Arrays for Peripheral Nerve Stimulation
Abstract
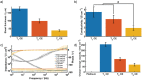
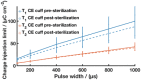
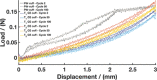
There is a critical need to transition research level flexible polymer bioelectronics toward the clinic by demonstrating both reliability in fabrication and stable device performance. Conductive elastomers (CEs) are composites of conductive polymers in elastomeric matrices that provide both flexibility and enhanced electrochemical properties compared to conventional metallic electrodes. This work focuses on the development of nerve cuff devices and the assessment of the device functionality at each development stage, from CE material to fully polymeric electrode arrays. Two device types are fabricated by laser machining of a thick and thin CE sheet variant on an insulative polydimethylsiloxane substrate and lamination into tubing to produce pre-curled cuffs. Device performance and stability following sterilization and mechanical loading are compared to a state-of-the-art stretchable metallic nerve cuff. The CE cuffs are found to be electrically and mechanically stable with improved charge transfer properties compared to the commercial cuff. All devices are applied to an ex vivo whole sciatic nerve and shown to be functional, with the CE cuffs demonstrating superior charge transfer and electrochemical safety in the biological environment.
Keywords: conductive elastomer; conductive polymer; electrode characterization; flexible bioelectronics; laser manufacturing; peripheral nerve cuff.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
