CRISPR/Cas9 Ribonucleoprotein-Based Genome Editing Methodology in the Marine Protozoan Parasite Perkinsus marinus
- PMID: 33898400
- PMCID: PMC8062965
- DOI: 10.3389/fbioe.2021.623278
CRISPR/Cas9 Ribonucleoprotein-Based Genome Editing Methodology in the Marine Protozoan Parasite Perkinsus marinus
Abstract
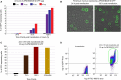
Perkinsus marinus (Perkinsozoa), a close relative of apicomplexans, is an osmotrophic facultative intracellular marine protozoan parasite responsible for "Dermo" disease in oysters and clams. Although there is no clinical evidence of this parasite infecting humans, HLA-DR40 transgenic mice studies strongly suggest the parasite as a natural adjuvant in oral vaccines. P. marinus is being developed as a heterologous gene expression platform for pathogens of medical and veterinary relevance and a novel platform for delivering vaccines. We previously reported the transient expression of two rodent malaria genes Plasmodium berghei HAP2 and MSP8. In this study, we optimized the original electroporation-based protocol to establish a stable heterologous expression method. Using 20 μg of pPmMOE[MOE1]:GFP and 25.0 × 106 P. marinus cells resulted in 98% GFP-positive cells. Furthermore, using the optimized protocol, we report for the first time the successful knock-in of GFP at the C-terminus of the PmMOE1 using ribonucleoprotein (RNP)-based CRISPR/Cas9 gene editing methodology. The GFP was expressed 18 h post-transfection, and expression was observed for 8 months post-transfection, making it a robust and stable knock-in system.
Keywords: CRISPR/Cas9; Perkinsus marinus; heterologous expression system; oral adjuvant; oral vaccines; protozoan; transfection.
Copyright © 2021 Yadavalli, Umeda, Waugh, Tracy, Sidhu, Hernández and Fernández Robledo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Transient Expression of Plasmodium berghei MSP8 and HAP2 in the Marine Protozoan Parasite Perkinsus marinus.J Parasitol. 2017 Feb;103(1):118-122. doi: 10.1645/16-88. Epub 2016 Oct 10. J Parasitol. 2017. PMID: 27723436 Free PMC article.
-
Humanized HLA-DR4 mice fed with the protozoan pathogen of oysters Perkinsus marinus (Dermo) do not develop noticeable pathology but elicit systemic immunity.PLoS One. 2014 Jan 31;9(1):e87435. doi: 10.1371/journal.pone.0087435. eCollection 2014. PLoS One. 2014. PMID: 24498105 Free PMC article.
-
Transfection of the protozoan parasite Perkinsus marinus.Mol Biochem Parasitol. 2008 Jan;157(1):44-53. doi: 10.1016/j.molbiopara.2007.09.007. Epub 2007 Oct 2. Mol Biochem Parasitol. 2008. PMID: 17996961
-
Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing.Theranostics. 2021 Jan 1;11(2):614-648. doi: 10.7150/thno.47007. eCollection 2021. Theranostics. 2021. PMID: 33391496 Free PMC article. Review.
-
CRISPR/Cas9 Delivery System Engineering for Genome Editing in Therapeutic Applications.Pharmaceutics. 2021 Oct 9;13(10):1649. doi: 10.3390/pharmaceutics13101649. Pharmaceutics. 2021. PMID: 34683943 Free PMC article. Review.
Cited by
-
Development of a novel electroporation method for the oyster parasite Perkinsus marinus.Sci Rep. 2022 Nov 21;12(1):19996. doi: 10.1038/s41598-022-24548-0. Sci Rep. 2022. PMID: 36411330 Free PMC article.
-
Perkinsus marinus in bioreactor: growth and a cost-reduced growth medium.J Ind Microbiol Biotechnol. 2023 Feb 17;50(1):kuad023. doi: 10.1093/jimb/kuad023. J Ind Microbiol Biotechnol. 2023. PMID: 37669897 Free PMC article.
-
CRISPR/Cas9-induced disruption of Bodo saltans paraflagellar rod-2 gene reveals its importance for cell survival.Environ Microbiol. 2022 Jul;24(7):3051-3062. doi: 10.1111/1462-2920.15918. Epub 2022 Feb 2. Environ Microbiol. 2022. PMID: 35099107 Free PMC article.
References
-
- Andrews J. D. (1996). History of Perkinsus marinus, a pathogen of oysters in Chesapeake Bay 1950-1984. J. Shellfish Res. 15 13–16.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

