Illuminating the "Black Box" of Progesterone-Dependent Embryo Implantation Using Engineered Mice
- PMID: 33898429
- PMCID: PMC8058370
- DOI: 10.3389/fcell.2021.640907
Illuminating the "Black Box" of Progesterone-Dependent Embryo Implantation Using Engineered Mice
Abstract
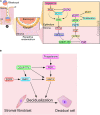
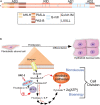
Synchrony between progesterone-driven endometrial receptivity and the arrival of a euploid blastocyst is essential for embryo implantation, a prerequisite event in the establishment of a successful pregnancy. Advancement of embryo implantation within the uterus also requires stromal fibroblasts of the endometrium to transform into epithelioid decidual cells, a progesterone-dependent cellular transformation process termed decidualization. Although progesterone is indispensable for these cellular processes, the molecular underpinnings are not fully understood. Because human studies are restricted, much of our fundamental understanding of progesterone signaling in endometrial periimplantation biology comes from in vitro and in vivo experimental systems. In this review, we focus on the tremendous progress attained with the use of engineered mouse models together with high throughput genome-scale analysis in disclosing key signals, pathways and networks that are required for normal endometrial responses to progesterone during the periimplantation period. Many molecular mediators and modifiers of the progesterone response are implicated in cross talk signaling between epithelial and stromal cells of the endometrium, an intercellular communication system that is critical for the ordered spatiotemporal control of embryo invasion within the maternal compartment. Accordingly, derailment of these signaling systems is causally linked with infertility, early embryo miscarriage and gestational complications that symptomatically manifest later in pregnancy. Such aberrant progesterone molecular responses also contribute to endometrial pathologies such as endometriosis, endometrial hyperplasia and cancer. Therefore, our review makes the case that further identification and functional analysis of key molecular mediators and modifiers of the endometrial response to progesterone will not only provide much-needed molecular insight into the early endometrial cellular changes that promote pregnancy establishment but lend credible hope for the development of more effective mechanism-based molecular diagnostics and precision therapies in the clinical management of female infertility, subfertility and a subset of gynecological morbidities.
Keywords: decidualization; endometrium; human; implantation; mediators and modifiers; mouse; progesterone; receptivity.
Copyright © 2021 Maurya, DeMayo and Lydon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Attar E., Tokunaga H., Imir G., Yilmaz M. B., Redwine D., Putman M., et al. (2009). Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J. Clin. Endocrinol. Metab. 94 623–631. 10.1210/jc.2008-1180 - DOI - PMC - PubMed
-
- Bain D. L., Franden M. A., Mcmanaman J. L., Takimoto G. S., Horwitz K. B. (2000). The N-terminal region of the human progesterone A-receptor. Structural analysis and the influence of the DNA binding domain. J. Biol. Chem. 275 7313–7320. - PubMed
-
- Bellofiore N., Ellery S. J., Mamrot J., Walker D. W., Temple-Smith P., Dickinson H. (2017). First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus). Am. J. Obstet. Gynecol. 216 40.e1–40.e11. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

