Long Non-coding RNAs and MicroRNAs Interplay in Osteogenic Differentiation of Mesenchymal Stem Cells
- PMID: 33898434
- PMCID: PMC8063120
- DOI: 10.3389/fcell.2021.646032
Long Non-coding RNAs and MicroRNAs Interplay in Osteogenic Differentiation of Mesenchymal Stem Cells
Abstract
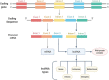
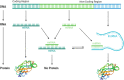
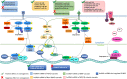
Long non-coding RNAs (lncRNAs) have gained great attention as epigenetic regulators of gene expression in many tissues. Increasing evidence indicates that lncRNAs, together with microRNAs (miRNAs), play a pivotal role in osteogenesis. While miRNA action mechanism relies mainly on miRNA-mRNA interaction, resulting in suppressed expression, lncRNAs affect mRNA functionality through different activities, including interaction with miRNAs. Recent advances in RNA sequencing technology have improved knowledge into the molecular pathways regulated by the interaction of lncRNAs and miRNAs. This review reports on the recent knowledge of lncRNAs and miRNAs roles as key regulators of osteogenic differentiation. Specifically, we described herein the recent discoveries on lncRNA-miRNA crosstalk during the osteogenic differentiation of mesenchymal stem cells (MSCs) derived from bone marrow (BM), as well as from different other anatomical regions. The deep understanding of the connection between miRNAs and lncRNAs during the osteogenic differentiation will strongly improve knowledge into the molecular mechanisms of bone growth and development, ultimately leading to discover innovative diagnostic and therapeutic tools for osteogenic disorders and bone diseases.
Keywords: crosstalk; interplay; lncRNA; long non-coding RNA; mesenchymal stem cell; miRNA; microRNA; osteogenic differentiation.
Copyright © 2021 Lanzillotti, De Mattei, Mazziotta, Taraballi, Rotondo, Tognon and Martini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Barbanti Bròdano G., Griffoni C., Nataloni A., Manfrini M., Giavaresi G., Bandiera S., et al. (2017). Biomaterials as bone graft substitutes for spine surgery: from preclinical results to clinical study. J. Biol. Regul. Homeost. Agents 31 167–181. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

