Changes in Cell Vitality, Phenotype, and Function of Dromedary Camel Leukocytes After Whole Blood Exposure to Heat Stress in vitro
- PMID: 33898545
- PMCID: PMC8062783
- DOI: 10.3389/fvets.2021.647609
Changes in Cell Vitality, Phenotype, and Function of Dromedary Camel Leukocytes After Whole Blood Exposure to Heat Stress in vitro
Abstract
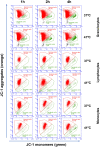
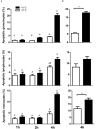
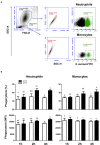
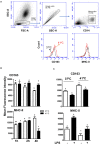
The dromedary camel (Camelus dromedarius) is well-adapted to the desert environment with the ability to tolerate increased internal body temperatures rising daily to 41-42°C during extreme hot. This study was undertaken to assess whether in vitro incubation of camel blood at 41°C, simulating conditions of heat stress, differently alters cell vitality, phenotype, and function of leukocytes, compared to incubation at 37°C (normothermia). Using flow cytometry, the cell vitality (necrosis and apoptosis), the expression of several cell markers and adhesion molecules, and the antimicrobial functions of camel leukocytes were analyzed in vitro. The fraction of apoptotic cells within the granulocytes, lymphocytes, and monocytes increased significantly after incubation of camel whole blood at 41°C for 4 h. The higher increase in apoptotic granulocytes and monocytes compared to lymphocytes suggests higher resistance of camel lymphocytes to heat stress. Functionally, incubation of camel blood at 41°C for 4 h enhanced the phagocytosis and ROS production activities of camel neutrophils and monocytes toward S. aureus. Monocytes from camel blood incubated at 41°C for 4 h significantly decreased their expression level of MHC class II molecules with no change in the abundance of CD163, resulting in a CD163high MHC-IIlow M2-like macrophage phenotype. In addition, heat stress treatment showed an inhibitory effect on the LPS-induced changes in camel monocytes phenotype. Furthermore, in vitro incubation of camel blood at 41°C reduced the expression of the cell adhesion molecules CD18 and CD11a on neutrophils and monocytes. Collectively, the present study identified some heat-stress-induced phenotypic and functional alterations in camel blood leukocytes, providing a paradigm for comparative immunology in the large animals. The clinical relevance of the observed changes in camel leukocytes for the adaptation of the camel immune response to heat stress conditions needs further in vitro and in vivo studies.
Keywords: ROS; camel (Camelus dromedarius); heat stress; immunity; leukocytes; phagocytosis.
Copyright © 2021 Hussen.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Immunomodulatory Effects of the Cyclooxygenase Inhibitor Lornoxicam on Phenotype and Function of Camel Blood Leukocytes.Animals (Basel). 2021 Jul 6;11(7):2023. doi: 10.3390/ani11072023. Animals (Basel). 2021. PMID: 34359151 Free PMC article.
-
Bacterial species-specific modulatory effects on phenotype and function of camel blood leukocytes.BMC Vet Res. 2021 Jul 12;17(1):241. doi: 10.1186/s12917-021-02939-1. BMC Vet Res. 2021. PMID: 34247606 Free PMC article.
-
Dromedary camel CD14high MHCIIhigh monocytes display inflammatory properties and are reduced in newborn camel calves.BMC Vet Res. 2020 Feb 18;16(1):62. doi: 10.1186/s12917-020-02285-8. BMC Vet Res. 2020. PMID: 32070351 Free PMC article.
-
Recent Advances in Camel Immunology.Front Immunol. 2021 Jan 25;11:614150. doi: 10.3389/fimmu.2020.614150. eCollection 2020. Front Immunol. 2021. PMID: 33569060 Free PMC article.
-
Cellular and Molecular Adaptation of Arabian Camel to Heat Stress.Front Genet. 2019 Jun 19;10:588. doi: 10.3389/fgene.2019.00588. eCollection 2019. Front Genet. 2019. PMID: 31275361 Free PMC article. Review.
Cited by
-
Association of Environmental Temperature and Relative Humidity with Ocular and Flank Temperatures in Dromedary Camels.Animals (Basel). 2025 Jan 22;15(3):309. doi: 10.3390/ani15030309. Animals (Basel). 2025. PMID: 39943079 Free PMC article.
-
The Impact of the Animal Housing System on Immune Cell Composition and Function in the Blood of Dromedary Camels.Animals (Basel). 2022 Jan 28;12(3):317. doi: 10.3390/ani12030317. Animals (Basel). 2022. PMID: 35158641 Free PMC article.
-
The Impact of Anticoagulation Agent on the Composition and Phenotype of Blood Leukocytes in Dromedary Camels.Vet Sci. 2022 Feb 13;9(2):78. doi: 10.3390/vetsci9020078. Vet Sci. 2022. PMID: 35202331 Free PMC article.
-
Serum cortisol level as marker of stress in camels: relationship with immunological profile.Front Vet Sci. 2025 Mar 25;12:1570564. doi: 10.3389/fvets.2025.1570564. eCollection 2025. Front Vet Sci. 2025. PMID: 40201077 Free PMC article.
-
Flow cytometric analysis of immune cell populations in the bronchial and mesenteric lymph nodes of the dromedary camel.Front Vet Sci. 2024 Apr 30;11:1365319. doi: 10.3389/fvets.2024.1365319. eCollection 2024. Front Vet Sci. 2024. PMID: 38746932 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

