Exploring the molecular structure, vibrational spectroscopic, quantum chemical calculation and molecular docking studies of curcumin: A potential PI3K/AKT uptake inhibitor
- PMID: 33898809
- PMCID: PMC8056428
- DOI: 10.1016/j.heliyon.2021.e06646
Exploring the molecular structure, vibrational spectroscopic, quantum chemical calculation and molecular docking studies of curcumin: A potential PI3K/AKT uptake inhibitor
Abstract
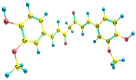
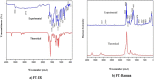
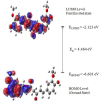
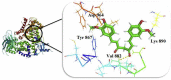
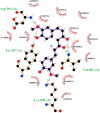
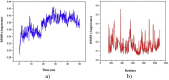
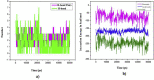
The IUPAC name of curcumin is (1E, 6E)-1,7-Bis(4-hydroxy-3methoxyphenyl) hepta-1,6-e-3,5-dione (7B3M5D) and is characterized by spectroscopic profiling with FT-IR and FT-Raman spectra obtained both experimentally and theoretically. PED analysis was done for the confirmation of minimum energy obtained in the title compound. Optimized geometrical parameters are compared with experimental values obtained for 7B3M5D by utilizing B3LYP functional employing 6-311++G (d,p) level of theory. The HOMO-LUMO, MEP, and Fukui function analysis has been used to elucidate the information regarding charge transfer within the molecule. The stabilization energy and charge delocalization of the 7B3M5D were performed by NBO analysis. This article assesses that the title compound act as a potential inhibitor of the PI3K/AKT inhibitor through in silico studies, like molecular docking, molecular dynamics (MD), ADMET prediction and also this molecule obeys Lipinski's rule of five. 7B3M5D was docked effectively in the active site of PI3K/AKT inhibitor.
Keywords: ADMET prediction; Docking; FT-IR; FT-Raman; Molecular dynamics and molecular.
© 2021 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. Ca - Cancer J. Clin. 2011;61:69–90. - PubMed
-
- Vanhaesebroeck B., Stephens L., Hawkins P. PI3K signaling the path to discovery and understanding. Nat. Rev. Mol. Cell Biol. 2012;13:195–203. - PubMed
-
- Mayer I.A., Arteaga C.L. The PI3K/AKT pathway as a target for cancer treatment. Annu. Rev. Med. 2016;67:11–28. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

