The New EU Regulation on In Vitro Diagnostic Medical Devices: Implications and Preparatory Actions for Diagnostic Laboratories
- PMID: 33898932
- PMCID: PMC8061679
- DOI: 10.1097/HS9.0000000000000568
The New EU Regulation on In Vitro Diagnostic Medical Devices: Implications and Preparatory Actions for Diagnostic Laboratories
Figures

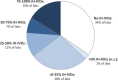
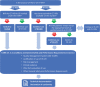
References
-
- Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. 2017. Available at: https://eur-lex.europa.eu/eli/reg/2017/746. Accessed April 8, 2021.
-
- Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. 1998. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31998L0079. Accessed April 8, 2021.
-
- van Drongelen A, de Bruijn A, Pennings J, van der Maaden T. The impact of the new European IVD classification rules on the notified body involvement; a study on the IVDs registered in the Netherlands. RIVM Letter Report. 2018;2018-0082:1–32.
-
- MedTech Europe. Clinical Evidence Requirements for CE certification under the in-vitro Diagnostic Regulation in the European Union. 2020. Available at: https://www.medtecheurope.org/resource-library/clinical-evidence-require.... Accessed March 12, 2021.
-
- European Commission. Nando (New Approach Notified and Designated Organisations) Information System: Notified bodies designated for the IVDR. Available at: https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=d.... Accessed March 12, 2021.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
