Weight-loss-independent benefits of exercise on liver steatosis and stiffness in Japanese men with NAFLD
- PMID: 33898958
- PMCID: PMC8059085
- DOI: 10.1016/j.jhepr.2021.100253
Weight-loss-independent benefits of exercise on liver steatosis and stiffness in Japanese men with NAFLD
Abstract
Background & aims: A weight-loss-independent beneficial effect of exercise on non-alcoholic fatty liver disease (NAFLD) management has been reported, but the underlying mechanism is unknown. To help determine this mechanism, the effects of exercise on individual tissues (liver, adipose tissue, and skeletal muscle) were retrospectively studied.
Methods: Data from Japanese obese men with NAFLD in a 3-month exercise regimen were analysed and compared with those in a 3-month dietary restriction program designed to achieve weight loss. The underlying mechanism was studied in a smaller subcohort.
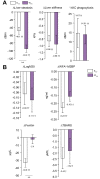
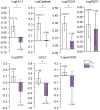
Results: Independent of the effect of weight loss, the exercise regimen reduced liver steatosis by 9.5% and liver stiffness by 6.8% per 1% weight loss, and resulted in a 16.4% reduction in FibroScan-AST score. Improvements in these hepatic parameters were closely associated with anthropometric changes (reduction in adipose tissue and preservation of muscle mass), increases in muscle strength (+11.6%), reductions in inflammation and oxidative stress (ferritin: -22.3% and thiobarbituric acid: -12.3%), and changes in organokine concentrations (selenoprotein-P: -11.2%, follistatin: +17.1%, adiponectin: +8.9%, and myostatin: -21.6%) during the exercise regimen. Moreover, the expression of target genes of the transcription factor Nrf2, an oxidative stress sensor, was higher in monocytes, suggesting that Nrf2 is activated. Large amounts of high-intensity exercise were effective at further reducing liver steatosis and potentiating improvements in pathophysiological parameters (liver enzyme activities and organokine profiles).
Conclusions: The weight-loss-independent benefits of exercise include anti-steatotic and anti-stiffness effects in the livers of patients with NAFLD. These benefits seem to be acquired through the modification of inter-organ crosstalk, which is characterised by improvements in organokine imbalance and reductions in inflammation and oxidative stress.
Lay summary: We investigated the effects of exercise on non-alcoholic fatty liver disease (NAFLD) that were not related to weight loss. We found that exercise had considerable weight-loss-independent benefits for the liver through a number of mechanisms. This suggests that exercise is important for NAFLD patients, regardless of whether they lose weight.
Keywords: ALT, alanine aminotransferase; ANGPTL6, angiopoietin-like 6; AST, aspartate aminotransferase; Aerobic exercise; BDNF, brain-derived neurotrophic factor; CAP, controlled attenuation parameter; Dietary restriction; Elarge, large amount of exercise group; Esmall, small amount of exercise group; Esub, exercise (subset for which biological samples were available) group; Etotal, exercise group; FAST-Score, FibroScan-AST score; FGF-21, fibroblast growth factor-21; FPG, fasting plasma glucose; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; GGT, gamma-glutamyl transpeptidase; GPx, glutathione peroxidase; HO1, heme oxygenase 1; HOMA-IR, homeostasis model assessment-insulin resistance; Hepatokine; KC, Kupffer cells; LPS, lipopolysaccharide; LSM, liver stiffness measured using transient elastography; Liver fat; Liver stiffness; MVPA, moderate-to-vigorous intensity physical activity; Myokine; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NEFAs, non-esterified fatty acids; NF-Score, NAFLD fibrosis score; NQO1, NAD(P)H quinone oxidoreductase; Nrf2, nuclear factor E2-related factor 2; Nuclear factor-erythroid 2-related factor 2; PBMCs, peripheral blood mononuclear cells; SPARC, secreted protein acidic and rich in cysteine; Se-P, selenoprotein-P; TBARS, thiobarbituric acid-reactive substances; TEI, total energy intake; TG, triglycerides; TNF-α, tumour necrosis factor alpha; VAT, visceral adipose tissue; WC, waist circumference; WFA+-M2BP, Wisteria floribunda agglutinin-positive human Mac-2 binding protein; Wsub, weight-loss (subset for which biological samples were available) group; Wtotal, weight-loss group; mnSOD, manganese superoxide dismutase.
© 2021 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- Eguchi Y., Hyogo H., Ono M., Mizuta T., Ono N., Fujimoto K. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–595. - PubMed
-
- Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

