Effects of a multi-faceted education and support programme on anxiety symptoms among people with systemic sclerosis and anxiety during COVID-19 (SPIN-CHAT): a two-arm parallel, partially nested, randomised, controlled trial
- PMID: 33899008
- PMCID: PMC8051930
- DOI: 10.1016/S2665-9913(21)00060-6
Effects of a multi-faceted education and support programme on anxiety symptoms among people with systemic sclerosis and anxiety during COVID-19 (SPIN-CHAT): a two-arm parallel, partially nested, randomised, controlled trial
Abstract
Background: No trials have tested multifaceted mental health interventions recommended by public health organisations during COVID-19. The objective of this trial was to evaluate the effect of the Scleroderma Patient-centered Intervention Network COVID-19 Home-isolation Activities Together (SPIN-CHAT) Program on anxiety symptoms and other mental health outcomes among people vulnerable during COVID-19 owing to a pre-existing medical condition.
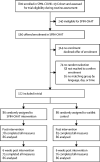
Methods: The SPIN-CHAT Trial was a pragmatic, two-arm, parallel, partially nested, randomised, controlled trial (1:1 allocation to intervention or waitlist). Eligible participants with systemic sclerosis were recruited from the international SPIN COVID-19 Cohort. SPIN COVID-19 Cohort participants were eligible for the trial if they completed baseline measures and had at least mild anxiety symptoms, had not tested positive for COVID-19, and were not currently receiving mental health counselling. SPIN-CHAT is a 4-week (3 sessions per week) videoconference-based group intervention that provided education and practice with mental health coping strategies, and provided social support to reduce isolation. Groups included 6-10 participants. The primary outcome analysed in the intention-to-treat population was anxiety symptoms (PROMIS Anxiety 4a version 1.0) immediately post-intervention. This trial is registered with ClinicalTrials.gov, NCT04335279 and is complete.
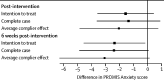
Findings: Of participants who completed baseline measures between April 9, 2020, and April 27, 2020, 560 participants were eligible and 172 participants were randomly assigned to intervention (n=86) or waitlist (n=86). Mean age was 55·0 years (SD 11·4 years), 162 (94%) were women, and 136 (79%) identified as White. In intention-to-treat analyses, the intervention did not significantly reduce anxiety symptoms post-intervention (-1·57 points, 95% CI -3·59 to 0·45; standardised mean difference [SMD] -0·22 points) but reduced symptoms 6 weeks later (-2·36 points, 95% CI -4·56 to -0·16; SMD -0·31). Depression symptoms were significantly lower 6 weeks post-intervention (-1·64 points, 95% CI -2·91 to -0·37; SMD -0·31); no other secondary outcomes were significant. No adverse events were reported.
Interpretation: The intervention did not significantly improve anxiety symptoms or other mental health outcomes post-intervention. However, anxiety and depression symptoms were significantly lower 6 weeks later, potentially capturing the time it took for new skills and social support between intervention participants to affect mental health. Multi-faceted interventions such as SPIN-CHAT have potential to address mental health needs in vulnerable groups during COVID-19, yet uncertainty remains about effectiveness.
Funding: Canadian Institutes of Health Research (CIHR; VR4-172745, MS1-173066); McGill Interdisciplinary Initiative in Infection and Immunity Emergency COVID-19 Research Fund; Scleroderma Canada, made possible by an educational grant for patient support programming from Boehringer Ingelheim; the Scleroderma Society of Ontario; Scleroderma Manitoba; Scleroderma Atlantic; Scleroderma Australia; Scleroderma New South Wales; Scleroderma Victoria; Scleroderma Queensland; Scleroderma SASK; the Scleroderma Association of BC; and Sclérodermie Québec.
© 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form. LM reported personal fees from Actelion–Johnson & Johnson, grants from LFB, non-financial support from Octapharma, and non-financial support from Grifols, all outside the submitted work. All other authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years. All authors declare no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/
-
- WHO Impact of COVID-19 on people's livelihoods, their health and our food systems: joint statement by ILO, FAO, IFAD and WHO. https://www.who.int/news/item/13-10-2020-impact-of-covid-19-on-people%27...
-
- DEPRESSD Living systematic review of mental health in COVID-19. https://www.depressd.ca/covid-19-mental-health
-
- WHO Mental health considerations during COVID-19 outbreak. https://www.who.int/docs/default-source/coronaviruse/mental-health-consi...
-
- Public Health England Guidance for the public on the mental health and wellbeing aspects of coronavirus (COVID-19) https://www.gov.uk/government/publications/covid-19-guidance-for-the-pub...
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

