Current state of commercial point-of-care nucleic acid tests for infectious diseases
- PMID: 33899053
- PMCID: PMC8139840
- DOI: 10.1039/d0an01988g
Current state of commercial point-of-care nucleic acid tests for infectious diseases
Abstract
The COVID-19 pandemic has put the spotlight on the urgent need for integrated nucleic acid tests (NATs) for infectious diseases, especially those that can be used near patient ("point-of-care", POC), with rapid results and low cost, but without sacrificing sensitivity or specificity of gold standard PCR tests. In the US, the Clinical Laboratory Improvement Amendments Certificate of Waiver (CLIA-waiver) is mandated by the Food and Drug Administration (FDA) and designated to any laboratory testing with high simplicity and low risk for error, suitable for application in the POC. Since the first issuance of CLIA-waiver to Abbot's ID NOW Influenza A&B in 2015, many more NAT systems have been developed, received the CLIA-waiver in the US or World Health Organization (WHO)'s pre-qualification, and deployed to the front line of infectious disease detection. This review highlights the regulatory process for FDA and WHO in evaluating these NATs and the technology innovation of existing CLIA-waived systems. Understanding the technical advancement and challenges, unmet needs, and the trends of commercialization facilitated through the regulatory processes will help pave the foundation for future development and technology transfer from research to the market place.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures

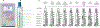

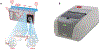
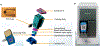



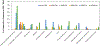
References
-
- United States Patent, US4683195A, Process for amplifying, detecting, and/or-cloning nucleic acid sequences, 1987.
-
- Yolken RH, in Methods in Enzymology, eds. Wilchek M and Bayer EA, Academic Press, 1990, vol. 184, pp. 529–537.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

