Higher Medication Adherence and Lower Opioid Use Among Individuals with Autoimmune Disease Enrolled in an Adalimumab Patient Support Program in the United States
- PMID: 33899166
- PMCID: PMC8217395
- DOI: 10.1007/s40744-021-00309-9
Higher Medication Adherence and Lower Opioid Use Among Individuals with Autoimmune Disease Enrolled in an Adalimumab Patient Support Program in the United States
Abstract
Introduction: Opioid use is prevalent among patients with autoimmune conditions, despite not being a recommended treatment. Tumor necrosis factor inhibitor (anti-TNF) therapy is an effective treatment for these autoimmune conditions, and patient support programs (PSPs) have been developed to help patients manage their prescribed treatments. This study was conducted to evaluate the impact of PSPs on anti-TNF adherence and opioid use using data on adalimumab (ADA), an anti-TNF.
Methods: The study used insurance claims data linked to ADA PSP data on patients who initiated ADA after 01/2015, were commercially insured, and had data coverage for 1 year before and after (i.e., during the follow-up period) ADA initiation. Patients with opioid use in the 3 months before ADA initiation were excluded. PSP patients enrolled in the PSP within 30 days of ADA initiation and had 2+ PSP nurse ambassador interactions; non-PSP patients had no PSP engagement. ADA adherence [proportion of days covered (PDC), persistence], opioid initiation, 2+ opioid fills, and opioid supply during follow-up were compared between cohorts using regression models that controlled for patient characteristics.
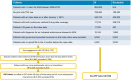
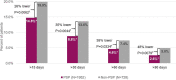
Results: Results were obtained for 1952 PSP and 728 non-PSP patients. PSP patients demonstrated better adherence to ADA than non-PSP patients, including higher PDC and persistence (all p < 0.001). PSP patients were 13% less likely to initiate opioids and 26% less likely to have at least 2 fills than non-PSP patients, and they had fewer days of opioid supply (all p < 0.01).
Conclusions: This study supports the benefit of PSPs and suggests that the ADA PSP is associated with improved adherence and potentially lower opioid use.
Keywords: Adalimumab; Autoimmune disorder; Opioid; Patient support program; Treatment adherence.
Figures

References
-
- US Department of Health and Human Services. HHS Acting Secretary declares public health emergency to address national opioid crisis. 2017. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-.... Accessed Sept 21, 2020.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

