A Nazarov-Ene Tandem Reaction for the Stereoselective Construction of Spiro Compounds
- PMID: 33899295
- PMCID: PMC8361765
- DOI: 10.1002/chem.202101041
A Nazarov-Ene Tandem Reaction for the Stereoselective Construction of Spiro Compounds
Abstract
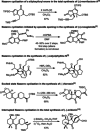
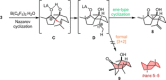
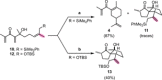
The different reactivity of trienones under Lewis and Brønsted acids catalysis was investigated, resulting in distinct cyclization products and carbon backbones that originated either from a conjugate Prins cyclization or an interrupted Nazarov cyclization. In particular, an unprecedented Nazarov cyclization tandem reaction is presented, terminating the oxyallyl cation by an ene-type reaction, and leading stereoselectively to bicyclic spiro compounds. The terminal olefin of this motif represents a useful handle for further functionalization, making it a strategic intermediate in total syntheses. The tandem Nazarov/ene cyclization was shown to be preferred over a Nazarov/[3+2] tandem reaction for all our substrates, independent of chain length. Deuteration studies further support the mechanistic hypothesis of the terminating ene reaction.
Keywords: Nazarov cyclization; cyclization; domino reactions; ene reaction; spiro compounds.
© 2021 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- He W., Huang J., Sun X., Frontier A. J., J. Am. Chem. Soc. 2007, 129, 498–499. - PubMed
-
- None
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

