Ambiphilic Al-Cu Bonding
- PMID: 33899319
- PMCID: PMC8252794
- DOI: 10.1002/anie.202104658
Ambiphilic Al-Cu Bonding
Abstract
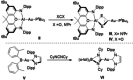
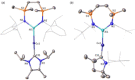
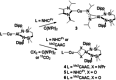
Copper-alumanyl complexes, [LCu-Al(SiNDipp )], where L=carbene=NHCiPr (N,N'-diisopropyl-4,5-dimethyl-2-ylidene) and Me2 CAAC (1-(2,6-diisopropylphenyl)-3,3,5,5-tetramethyl-pyrrolidin-2-ylidene) and featuring unsupported Al-Cu bonds, have been prepared. Divergent reactivity observed with carbodiimides and CO2 implies an ambiphilicity in the Cu-Al interaction that is dependent on the identity of the carbene co-ligand.
Keywords: aluminum; coordination modes; copper; ligand effects; metal-metal interactions.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
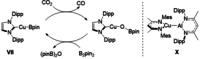



Similar articles
-
On the reactivity of Al-group 11 (Cu, Ag, Au) bonds.Dalton Trans. 2022 Mar 8;51(10):3913-3924. doi: 10.1039/d2dt00404f. Dalton Trans. 2022. PMID: 35169824
-
Tris(pentafluoroethyl)difluorophosphorane for fluoride abstraction and ligand exchange reactions of N-heterocyclic carbene and cyclic alkyl(amino)carbene copper(I) fluorides.Dalton Trans. 2025 Jun 10;54(23):9310-9328. doi: 10.1039/d5dt00904a. Dalton Trans. 2025. PMID: 40405796
-
Highly Reactive Cyclic(alkyl)(amino) Carbene- and N-Heterocyclic Carbene-Bismuth(III) Complexes: Synthesis, Structure, and Computations.Inorg Chem. 2018 Sep 17;57(18):11687-11695. doi: 10.1021/acs.inorgchem.8b01813. Epub 2018 Aug 30. Inorg Chem. 2018. PMID: 30160485
-
Activation of Ge-H and Sn-H Bonds with N-Heterocyclic Carbenes and a Cyclic (Alkyl)(amino)carbene.Chemistry. 2023 Jan 12;29(3):e202202493. doi: 10.1002/chem.202202493. Epub 2022 Nov 24. Chemistry. 2023. PMID: 36177710 Free PMC article.
-
Structural snapshots of an Al-Cu bond-mediated transformation of terminal acetylenes.Chem Sci. 2023 Feb 15;14(11):2866-2876. doi: 10.1039/d3sc00240c. eCollection 2023 Mar 15. Chem Sci. 2023. PMID: 36937577 Free PMC article.
Cited by
-
Inducing Nucleophilic Reactivity at Beryllium with an Aluminyl Ligand.J Am Chem Soc. 2023 Mar 1;145(8):4408-4413. doi: 10.1021/jacs.3c00480. Epub 2023 Feb 14. J Am Chem Soc. 2023. PMID: 36786728 Free PMC article.
-
Divergent CO2 Activation by Tuning the Lewis Acid in Iron-Based Bimetallic Systems.Angew Chem Int Ed Engl. 2022 Oct 4;61(40):e202207581. doi: 10.1002/anie.202207581. Epub 2022 Aug 29. Angew Chem Int Ed Engl. 2022. PMID: 35930523 Free PMC article.
-
Coinage metal aluminyl complexes: probing regiochemistry and mechanism in the insertion and reduction of carbon dioxide.Chem Sci. 2021 Sep 16;12(40):13458-13468. doi: 10.1039/d1sc04676d. eCollection 2021 Oct 20. Chem Sci. 2021. PMID: 34777765 Free PMC article.
-
Aluminum-Boron Bond Formation by Boron Ester Oxidative Addition at an Alumanyl Anion.Inorg Chem. 2023 Sep 18;62(37):15310-15319. doi: 10.1021/acs.inorgchem.3c02566. Epub 2023 Sep 6. Inorg Chem. 2023. PMID: 37672789 Free PMC article.
-
π-Bonding of Group 11 Metals to a Tantalum Alkylidyne Alkyl Complex Promotes Unusual Tautomerism to Bis-alkylidene and CO2 to Ketenyl Transformation.J Am Chem Soc. 2024 Jul 10;146(27):18306-18319. doi: 10.1021/jacs.4c02172. Epub 2024 Jun 27. J Am Chem Soc. 2024. PMID: 38936814 Free PMC article.
References
-
- Taylor M. J., Metal-to-Metal Bonded States of the Main Group Elements, Academic Press, London, 1975.
-
- Berry J. F., Lu C. C., Inorg. Chem. 2017, 56, 7577–7581. - PubMed
-
- Hicks J., Vasko P., Goicoechea J. M., Aldridge S., Nature 2018, 557, 92–95. - PubMed
-
- Hicks J., Vasko P., Goicoechea J. M., Aldridge S., Angew. Chem. Int. Ed. 2021, 60, 1702–1713; - PubMed
- Angew. Chem. 2021, 133, 1726–1737.
-
- Schwamm R. J., Anker M. D., Lein M., Coles M. P., Angew. Chem. Int. Ed. 2019, 58, 1489–1493; - PubMed
- Angew. Chem. 2019, 131, 1503–1507.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

