Allopurinol hepatotoxicity is associated with human leukocyte antigen Class I alleles
- PMID: 33899326
- PMCID: PMC8286350
- DOI: 10.1111/liv.14903
Allopurinol hepatotoxicity is associated with human leukocyte antigen Class I alleles
Abstract
Background/aims: Allopurinol can cause HLA class I-associated life-threatening severe skin reactions. However, HLA risk and association with clinical features in allopurinol hepatotoxicity are unknown.
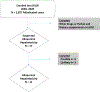
Methods: Eleven of 17 patients with suspected allopurinol hepatotoxicity enrolled into the Drug-Induced Liver Injury Network were adjudicated as definite, highly likely, or probable. High-resolution HLA sequencing was undertaken in cases and compared with population and other DILI controls.
Result: Median age was 60 years, 54% were male, and 63% African- American, 27% Caucasian, and 9% Hispanic. Patients presented at a median of 52 days after starting allopurinol, all were hospitalized and six were jaundiced. The median peak ALT, alkaline phosphatase, and total bilirubin were 525 U/L, 521 U/L, and 7.8 mg/dl, respectively, with a median R ratio of 2.7 at onset. During follow-up, nine patients were treated with corticosteroids including five of the six with suspected DRESS. Three patients died including two from liver failure at 38 and 45 days after onset, and the remaining eight recovered. Three HLA alleles were found to be overrepresented in allopurinol cases, particularly in African Americans: HLA-B*58:01, which has been previously linked to severe skin reactions, and HLA-B*53:01 and HLA-A*34:02, all of which are more frequently found in African Americans than European Americans or Latinos.
Conclusions: Allopurinol hepatotoxicity is associated with systemic hypersensitivity, a short latency to onset, African-American race and three HLA risk alleles, HLA-B*58:01, HLA-B*53:01, and HLA-A*34:02-58:01 testing may help confirm a diagnosis of hepatotoxicity in allopurinol-treated patients.
Keywords: genetic polymorphisms; gout; human leukocyte antigen; hyperuricaemia; liver injury.
© 2021 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
Similar articles
-
The role of HLA-A*33:01 in patients with cholestatic hepatitis attributed to terbinafine.J Hepatol. 2018 Dec;69(6):1317-1325. doi: 10.1016/j.jhep.2018.08.004. Epub 2018 Aug 21. J Hepatol. 2018. PMID: 30138689 Free PMC article.
-
Minocycline hepatotoxicity: Clinical characterization and identification of HLA-B∗35:02 as a risk factor.J Hepatol. 2017 Jul;67(1):137-144. doi: 10.1016/j.jhep.2017.03.010. Epub 2017 Mar 18. J Hepatol. 2017. PMID: 28323125 Free PMC article.
-
HLA alleles influence the clinical signature of amoxicillin-clavulanate hepatotoxicity.PLoS One. 2013 Jul 9;8(7):e68111. doi: 10.1371/journal.pone.0068111. Print 2013. PLoS One. 2013. PMID: 23874514 Free PMC article.
-
Pharmacogenomics of severe cutaneous adverse reactions and drug-induced liver injury.J Hum Genet. 2013 Jun;58(6):317-26. doi: 10.1038/jhg.2013.37. Epub 2013 May 2. J Hum Genet. 2013. PMID: 23635947 Review.
-
Drug eruptions induced by allopurinol associated with HLA-B*5801.Indian J Dermatol Venereol Leprol. 2015 Jan-Feb;81(1):43-5. doi: 10.4103/0378-6323.148566. Indian J Dermatol Venereol Leprol. 2015. PMID: 25566896 Review.
Cited by
-
Allopurinol: Clinical Considerations in the Development and Treatment of Stevens-Johnson Syndrome, Toxic Epidermal Necrolysis, and Other Associated Drug Reactions.Cureus. 2024 Jul 16;16(7):e64654. doi: 10.7759/cureus.64654. eCollection 2024 Jul. Cureus. 2024. PMID: 39149682 Free PMC article. Review.
-
Apigenin Ameliorates Hyperuricemia and Renal Injury through Regulation of Uric Acid Metabolism and JAK2/STAT3 Signaling Pathway.Pharmaceuticals (Basel). 2022 Nov 21;15(11):1442. doi: 10.3390/ph15111442. Pharmaceuticals (Basel). 2022. PMID: 36422572 Free PMC article.
-
Hepatotoxicity in inflammatory bowel disease: Immunomodulators, biologics, and beyond.Clin Liver Dis (Hoboken). 2024 Jun 14;23(1):e0199. doi: 10.1097/CLD.0000000000000199. eCollection 2024 Jan-Jun. Clin Liver Dis (Hoboken). 2024. PMID: 38881727 Free PMC article. No abstract available.
-
HLA-B*53:01 Is a Significant Risk Factor of Liver Injury due to Phenytoin and Other Antiepileptic Drugs in African Americans.Am J Gastroenterol. 2024 Jan 1;119(1):200-202. doi: 10.14309/ajg.0000000000002454. Epub 2023 Aug 8. Am J Gastroenterol. 2024. PMID: 37552102 Free PMC article.
-
HLA-B allele frequencies and implications for pharmacogenetics in the Kuwaiti population.Front Pharmacol. 2024 Oct 11;15:1423636. doi: 10.3389/fphar.2024.1423636. eCollection 2024. Front Pharmacol. 2024. PMID: 39464636 Free PMC article.
References
-
- Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005; 353: 2450–61 - PubMed
-
- Vanderstigel M, Zafrani ES, Lejonc JL, Schaeffer A, Portos JL. Allopurinol hypersensitivity syndrome as a cause of hepatic fibrin-ring granulomas. Gastroenterol 1986; 90: 188–90. - PubMed
-
- Khoo BP, Leow YH. A review of inpatients with adverse drug reactions to allopurinol. Singapore Med J 2000; 41: 156–60 - PubMed
-
- Kaniwa N, Saito Y, Aihara M, et al.; JSAR research group. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics 2008; 9: 1617–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U24 DK065176/DK/NIDDK NIH HHS/United States
- U01 DK083020/DK/NIDDK NIH HHS/United States
- U01 DK065238/DK/NIDDK NIH HHS/United States
- U01 DK065193/DK/NIDDK NIH HHS/United States
- U01 DK065176/DK/NIDDK NIH HHS/United States
- U01 DK083023/DK/NIDDK NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- UL1 RR024150/RR/NCRR NIH HHS/United States
- U01 DK065211/DK/NIDDK NIH HHS/United States
- U01 HG007417/HG/NHGRI NIH HHS/United States
- U01 DK083027/DK/NIDDK NIH HHS/United States
- U01 DK065201/DK/NIDDK NIH HHS/United States
- U01 DK100928/DK/NIDDK NIH HHS/United States
- UL1 TR000083/TR/NCATS NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- U01 DK065184/DK/NIDDK NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- U01 DK082992/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

