Generation of Stable Isopentenyl Monophosphate Aryloxy Triester Phosphoramidates as Activators of Vγ9Vδ2 T Cells
- PMID: 33899332
- PMCID: PMC8453817
- DOI: 10.1002/cmdc.202100198
Generation of Stable Isopentenyl Monophosphate Aryloxy Triester Phosphoramidates as Activators of Vγ9Vδ2 T Cells
Abstract
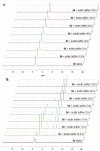
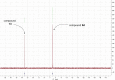
Aryloxy triester phosphoramidate prodrugs of the monophosphate derivatives of isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) were synthesized as lipophilic derivatives that can improve cell uptake. Despite the structural similarity of IPP and DMAPP, it was noted that their phosphoramidate prodrugs exhibited distinct stability profiles in aqueous environments, which we show is due to the position of the allyl bond in the backbones of the IPP and DMAPP monophosphates. As the IPP monophosphate aryloxy triester phosphoramidates showed favorable stability, they were subsequently investigated for their ability to activate Vγ9/Vδ2 T cells and they showed promising activation of this subset of T cells. Together, these findings represent the first report of IPP and DMAPP monophosphate prodrugs and the ability of IPP aryloxy triester phosphoramidate prodrugs to activate Vγ9/Vδ2 T cells highlighting their potential as possible immunotherapeutics.
Keywords: DMAPP; IPP; Phosphoramidates; Prodrugs; T-cells.
© 2021 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



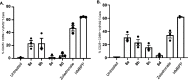
Similar articles
-
Synthesis and Biological Evaluation of ( E)-4-Hydroxy-3-methylbut-2-enyl Phosphate (HMBP) Aryloxy Triester Phosphoramidate Prodrugs as Activators of Vγ9/Vδ2 T-Cell Immune Responses.J Med Chem. 2018 Mar 8;61(5):2111-2117. doi: 10.1021/acs.jmedchem.7b01824. Epub 2018 Feb 23. J Med Chem. 2018. PMID: 29457898 Free PMC article.
-
Aryloxy Triester Phosphoramidates as Phosphoserine Prodrugs: A Proof of Concept Study.ChemMedChem. 2020 Apr 20;15(8):671-674. doi: 10.1002/cmdc.202000034. Epub 2020 Mar 26. ChemMedChem. 2020. PMID: 32162793
-
Synthesis and anti-herpetic activity of phosphoramidate ProTides.ChemMedChem. 2013 Jun;8(6):985-93. doi: 10.1002/cmdc.201300035. Epub 2013 Apr 18. ChemMedChem. 2013. PMID: 23606629
-
Phosphoramidates and phosphonamidates (ProTides) with antiviral activity.Antivir Chem Chemother. 2018 Jan-Dec;26:2040206618775243. doi: 10.1177/2040206618775243. Antivir Chem Chemother. 2018. PMID: 29792071 Free PMC article. Review.
-
Aryloxy phosphoramidate triesters as pro-tides.Mini Rev Med Chem. 2004 May;4(4):371-81. doi: 10.2174/1389557043403936. Mini Rev Med Chem. 2004. PMID: 15134540 Review.
References
-
- None
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

