[Evaluation on monitoring effect of the electronic vaccine vial monitor label]
- PMID: 33899440
- PMCID: PMC10307573
- DOI: 10.7507/1001-5515.202011038
[Evaluation on monitoring effect of the electronic vaccine vial monitor label]
Abstract
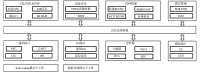
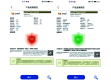
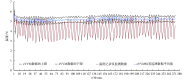
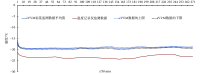
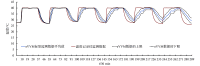
The cold chain safety of vaccines is a global issue. The electronic vaccine vial monitor (eVVM) label can monitor the temperature of vaccines in real time and provide "early warning" prompts. In order to comprehensively evaluate the monitoring efficiency of eVVM, this study selected 75 eVVM labels and distributed them with a total of 600 vaccine vial monitor (VVM) labels of four different types in different experimental environment (2-8℃, -20℃ and 40℃), and used a temperature recorder as "gold standard". The results showed that the accuracy of the eVVM labels and VVM labels in high temperature environment was as same as that of the temperature recorder ( P = 0.195). The accuracy of low temperature anomalies report and high temperature anomalies report of eVVM labels was 100%, which was better than those reported by VVM labels. Therefore, eVVM labels have high monitoring accuracy, which is suitable not only for ordinary environments, but also for severe temperature environments. It should be helpful for the improvement of the efficiency and accuracy of cold chain monitoring.
疫苗的冷链安全为全球性问题,电子疫苗热敏感标签(eVVM)可对疫苗温度进行实时监控,并进行“预警”提示。为综合评价 eVVM 的监测效能,本研究在不同产品批次中随机抽取 75 个 eVVM 标签,将其与四种不同类型共 600 个疫苗温度指示标签(VVM)平均分配到不同的实验环境中(2~8℃、−20℃ 以及 40℃),采用温度记录仪作为对照。结果显示,eVVM 标签、VVM 标签在高温温度监测方面的精确度与温度记录仪具有很好的一致性( P = 0.195),且 eVVM 标签在低温异常和高温异常的正确率均为 100%,优于 VVM 标签。因此,我们认为 eVVM 标签有较高的精确度,对于进一步提高冷链的监测效率及准确度均具有良好的应用前景。.
Keywords: label; thermal sensitivity; vaccine.
Figures

Similar articles
-
Application of the remaining vaccine vial monitor life calculation to field temperature monitoring data to improve visibility into cold chain equipment performance.Vaccine. 2020 Nov 10;38(48):7683-7687. doi: 10.1016/j.vaccine.2020.09.078. Epub 2020 Oct 17. Vaccine. 2020. PMID: 33082013
-
[Investigation on the cold-chain temperature of vaccine in some areas of Zhejiang Province].Zhonghua Yu Fang Yi Xue Za Zhi. 2018 Nov 6;52(11):1173-1176. doi: 10.3760/cma.j.issn.0253-9624.2018.11.015. Zhonghua Yu Fang Yi Xue Za Zhi. 2018. PMID: 30419704 Chinese.
-
[Study on the failure time of the vaccine vial monitor used for oral poliovirus vaccine at 25 ℃ and 37 ℃].Zhonghua Yu Fang Yi Xue Za Zhi. 2017 Mar 6;51(3):248-251. doi: 10.3760/cma.j.issn.0253-9624.2017.03.011. Zhonghua Yu Fang Yi Xue Za Zhi. 2017. PMID: 28260340 Chinese.
-
Tools and approaches to ensure quality of vaccines throughout the cold chain.Expert Rev Vaccines. 2014 Jul;13(7):843-54. doi: 10.1586/14760584.2014.923761. Epub 2014 May 28. Expert Rev Vaccines. 2014. PMID: 24865112 Free PMC article. Review.
-
Vaccine transport and storage: environmental challenges.Dev Biol Stand. 1996;87:9-17. Dev Biol Stand. 1996. PMID: 8853997 Review.
References
-
- 王月丹, 初明 疫苗接种安全问题的产生及其科学应对措施. 中国药物评价. 2014;31(1):56–60. doi: 10.3969/j.issn.2095-3593.2014.01.018. [王月丹, 初明. 疫苗接种安全问题的产生及其科学应对措施. 中国药物评价, 2014, 31(1): 56-60.] - DOI
-
- 姜晓飞 预防接种信息管理系统的实施及应用进展. 中国疫苗和免疫. 2014;20(1):79–84. [姜晓飞. 预防接种信息管理系统的实施及应用进展. 中国疫苗和免疫, 2014, 20(1): 79-84.]
-
- 应徐颉, 刘琼, 张明胜, 等 我国疫苗冷链管理现状及发展展望. 中国现代应用药学. 2020;37(5):636–640. [应徐颉, 刘琼, 张明胜, 等. 我国疫苗冷链管理现状及发展展望. 中国现代应用药学, 2020, 37(5): 636-640.]
-
- 田素霞 疫苗冷链运输过程中的质量风险管理研究. 中国当代医药. 2018;25(17):164–168. doi: 10.3969/j.issn.1674-4721.2018.17.050. [田素霞. 疫苗冷链运输过程中的质量风险管理研究. 中国当代医药, 2018, 25(17): 164-168.] - DOI
-
- 余文周, 王富珍, 温宁, 等 中国深化医疗改革十年免疫规划助力全民健康. 中国疫苗和免疫. 2020;26(5):602–606. [余文周, 王富珍, 温宁, 等. 中国深化医疗改革十年免疫规划助力全民健康. 中国疫苗和免疫, 2020, 26(5): 602-606.]
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
