Microphysiological systems: What it takes for community adoption
- PMID: 33899539
- PMCID: PMC8243212
- DOI: 10.1177/15353702211008872
Microphysiological systems: What it takes for community adoption
Abstract
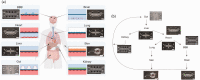
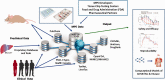
Microphysiological systems (MPS) are promising in vitro tools which could substantially improve the drug development process, particularly for underserved patient populations such as those with rare diseases, neural disorders, and diseases impacting pediatric populations. Currently, one of the major goals of the National Institutes of Health MPS program, led by the National Center for Advancing Translational Sciences (NCATS), is to demonstrate the utility of this emerging technology and help support the path to community adoption. However, community adoption of MPS technology has been hindered by a variety of factors including biological and technological challenges in device creation, issues with validation and standardization of MPS technology, and potential complications related to commercialization. In this brief Minireview, we offer an NCATS perspective on what current barriers exist to MPS adoption and provide an outlook on the future path to adoption of these in vitro tools.
Keywords: Microphysiological systems; National Institutes of Health; bioengineering; induced pluripotent stem cells; microfluidics.
Conflict of interest statement
Figures
References
-
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ 2016; 47:20–33 - PubMed
-
- Wagner J, Dahlem AM, Hudson LD, Terry SF, Altman RB, Gilliland CT, DeFeo C, Austin CP. A dynamic map for learning, communicating, navigating and improving therapeutic development. Nat Rev Drug Discov 2018; 17:150 - PubMed
-
- Arrowsmith J. Trial watch: phase II failures: 2008-2010. Nat Rev Drug Discov 2011; 10:328–9 - PubMed
-
- Arrowsmith J. Trial watch: phase III and submission failures: 2007-2010. Nat Rev Drug Discov 2011; 10:87. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

