Differential pial and penetrating arterial responses examined by optogenetic activation of astrocytes and neurons
- PMID: 33899558
- PMCID: PMC8504944
- DOI: 10.1177/0271678X211010355
Differential pial and penetrating arterial responses examined by optogenetic activation of astrocytes and neurons
Abstract
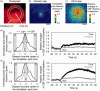
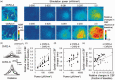
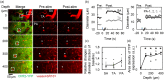
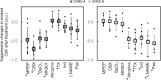
A variety of brain cells participates in neurovascular coupling by transmitting and modulating vasoactive signals. The present study aimed to probe cell type-dependent cerebrovascular (i.e., pial and penetrating arterial) responses with optogenetics in the cortex of anesthetized mice. Two lines of the transgenic mice expressing a step function type of light-gated cation channel (channelrhodopsine-2; ChR2) in either cortical neurons (muscarinic acetylcholine receptors) or astrocytes (Mlc1-positive) were used in the experiments. Photo-activation of ChR2-expressing astrocytes resulted in a widespread increase in cerebral blood flow (CBF), extending to the nonstimulated periphery. In contrast, photo-activation of ChR2-expressing neurons led to a relatively localized increase in CBF. The differences in the spatial extent of the CBF responses are potentially explained by differences in the involvement of the vascular compartments. In vivo imaging of the cerebrovascular responses revealed that ChR2-expressing astrocyte activation led to the dilation of both pial and penetrating arteries, whereas ChR2-expressing neuron activation predominantly caused dilation of the penetrating arterioles. Pharmacological studies showed that cell type-specific signaling mechanisms participate in the optogenetically induced cerebrovascular responses. In conclusion, pial and penetrating arterial vasodilation were differentially evoked by ChR2-expressing astrocytes and neurons.
Keywords: Animal model; cerebral microcirculation; in vivo optical imaging; laser speckle flowgraphy; two-photon microscopy.
Conflict of interest statement
Figures


References
-
- Lecrux C, Bourourou M, Hamel E. How reliable is cerebral blood flow to map changes in neuronal activity? Auton Neurosci 2019; 217: 71–79. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

