Connexin43 promotes angiogenesis through activating the HIF-1α/VEGF signaling pathway under chronic cerebral hypoperfusion
- PMID: 33899559
- PMCID: PMC8504949
- DOI: 10.1177/0271678X211010354
Connexin43 promotes angiogenesis through activating the HIF-1α/VEGF signaling pathway under chronic cerebral hypoperfusion
Abstract
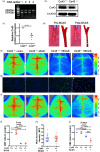
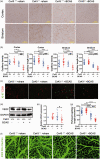
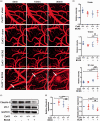
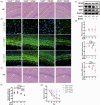
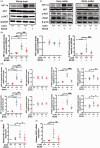
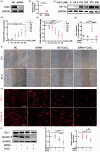
Chronic cerebral hypoperfusion, a major vascular contributor to vascular cognitive impairment and dementia, can exacerbate small vessel pathology. Connexin43, the most abundant gap junction protein in brain tissue, has been found to be critically involved in the pathological changes of vascular cognitive impairment and dementia caused by chronic cerebral hypoperfusion. However, the precise mechanisms underpinning its role are unclear. We established a mouse model via bilateral common carotid arteries stenosis on connexin43 heterozygous male mice and demonstrated that connexin43 improves brain blood flow recovery by mediating reparative angiogenesis under chronic cerebral hypoperfusion, which subsequently reduces the characteristic pathologies of vascular cognitive impairment and dementia including white matter lesions and irreversible neuronal injury. We additionally found that connexin43 mediates hypoxia inducible factor-1α expression and then activates the PKA signaling pathway to regulate vascular endothelial growth factor-induced angiogenesis. All the above findings were replicated in bEnd.3 cells treated with 375 µM CoCl2in vitro. These results suggest that connexin 43 could be instrumental in developing potential therapies for vascular cognitive impairment and dementia caused by chronic cerebral hypoperfusion.
Keywords: Chronic cerebral hypoperfusion; Connexin43; angiogenesis; hypoxia inducible factor-1α; vascular cognitive impairment and dementia.
Conflict of interest statement
Figures

References
-
- Toth P, Tarantini S, Csiszar A, et al.. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 2017; 312: H1–h20. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

