Access to Metal Centers and Fluxional Hydride Coordination Integral for CO2 Insertion into [Fe3(μ-H)3]3+ Clusters
- PMID: 33900076
- PMCID: PMC8247455
- DOI: 10.1021/acs.inorgchem.1c00244
Access to Metal Centers and Fluxional Hydride Coordination Integral for CO2 Insertion into [Fe3(μ-H)3]3+ Clusters
Abstract
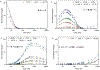
CO2 insertion into tri(μ-hydrido)triiron(II) clusters ligated by a tris(β-diketiminate) cyclophane is demonstrated to be balanced by sterics for CO2 approach and hydride accessibility. Time-resolved NMR and UV-vis spectra for this reaction for a complex in which methoxy groups border the pocket of the hydride donor (Fe3H3L2, 4) result in a decreased activation barrier and increased kinetic isotope effect consistent with the reduced sterics. For the ethyl congener Fe3H3L1 (2), no correlation is found between rate and reaction solvent or added Lewis acids, implying CO2 coordination to an Fe center in the mechanism. The estimated hydricity (50 kcal/mol) based on observed H/D exchange with BD3 requires Fe-O bond formation in the product to offset an endergonic CO2 insertion. μ3-hydride coordination is noted to lower the activation barrier for the first CO2 insertion event in DFT calculations.
Figures




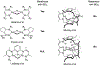

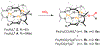


References
-
- Reijerse EJ; Pham CC; Pelmenschikov V; Gilbert-Wilson R; Adamska-Venkatesh A; Siebel JF; Gee LB; Yoda Y; Tamasaku K; Lubitz W; Rauchfuss TB; Cramer SP Direct Observation of an Iron-Bound Terminal Hydride in [FeFe]-Hydrogenase by Nuclear Resonance Vibrational Spectroscopy. J. Am. Chem. Soc 2017, 139 (12), 4306–4309. 10.1021/jacs.7b00686. - DOI - PMC - PubMed
-
- Appel AM; Bercaw JE; Bocarsly AB; Dobbek H; DuBois DL; Dupuis M; Ferry JG; Fujita E; Hille R; Kenis PJA; Kerfeld CA; Morris RH; Peden CHF; Portis AR; Ragsdale SW; Rauchfuss TB; Reek JNH; Seefeldt LC; Thauer RK; Waldrop GL Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev 2013, 113 (8), 6621–6658. 10.1021/cr300463y. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

