Sensing the world and its dangers: An evolutionary perspective in neuroimmunology
- PMID: 33900197
- PMCID: PMC8075586
- DOI: 10.7554/eLife.66706
Sensing the world and its dangers: An evolutionary perspective in neuroimmunology
Abstract
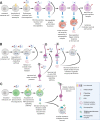
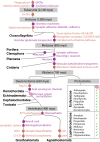
Detecting danger is key to the survival and success of all species. Animal nervous and immune systems cooperate to optimize danger detection. Preceding studies have highlighted the benefits of bringing neurons into the defense game, including regulation of immune responses, wound healing, pathogen control, and survival. Here, we summarize the body of knowledge in neuroimmune communication and assert that neuronal participation in the immune response is deeply beneficial in each step of combating infection, from inception to resolution. Despite the documented tight association between the immune and nervous systems in mammals or invertebrate model organisms, interdependence of these two systems is largely unexplored across metazoans. This review brings a phylogenetic perspective of the nervous and immune systems in the context of danger detection and advocates for the use of non-model organisms to diversify the field of neuroimmunology. We identify key taxa that are ripe for investigation due to the emergence of key evolutionary innovations in their immune and nervous systems. This novel perspective will help define the primordial principles that govern neuroimmune communication across taxa.
Keywords: evolution; immunology; inflammation; metazoans; nervous system; neuroimmunology; neuroscience; non-model organisms.
© 2021, Kraus et al.
Conflict of interest statement
AK, KB, IS No competing interests declared
Figures

References
-
- Alpizar YA, Boonen B, Sanchez A, Jung C, López-Requena A, Naert R, Steelant B, Luyts K, Plata C, De Vooght V, Vanoirbeek JAJ, Meseguer VM, Voets T, Alvarez JL, Hellings PW, Hoet PHM, Nemery B, Valverde MA, Talavera K. TRPV4 activation triggers protective responses to bacterial lipopolysaccharides in airway epithelial cells. Nature Communications. 2017;8:1059. doi: 10.1038/s41467-017-01201-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

