Sex Disparities in Enrollment in Recent Randomized Clinical Trials of Acute Stroke: A Meta-analysis
- PMID: 33900363
- PMCID: PMC8077045
- DOI: 10.1001/jamaneurol.2021.0873
Sex Disparities in Enrollment in Recent Randomized Clinical Trials of Acute Stroke: A Meta-analysis
Abstract
Importance: The underenrollment of women in randomized clinical trials represents a threat to the validity of the evidence supporting clinical guidelines and potential disparities in access to novel treatments.
Objective: To determine whether women were underenrolled in contemporary randomized clinical trials of acute stroke therapies published in 9 major journals after accounting for their representation in underlying stroke populations.
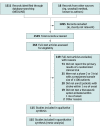
Data sources: MEDLINE was searched for acute stroke therapeutic trials published between January 1, 2010, and June 11, 2020.
Study selection: Eligible articles reported the results of a phase 2 or 3 randomized clinical trial that enrolled patients with stroke and/or transient ischemic attack and examined a therapeutic intervention initiated within 1 month of onset.
Data extraction: Data extraction was performed by 2 independent authors in duplicate. Individual trials were matched to estimates of the proportion of women in underlying stroke populations using the Global Burden of Disease database.
Main outcomes and measures: The primary outcome was the enrollment disparity difference (EDD), the absolute difference between the proportion of trial participants who were women and the proportion of strokes in the underlying disease populations that occurred in women. Random-effects meta-analyses of the EDD were performed, and multivariable metaregression was used to explore the associations of trial eligibility criteria with disparity estimates.
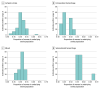
Results: The search returned 1529 results, and 115 trials (7.5%) met inclusion criteria. Of 121 105 randomized patients for whom sex was reported, 52 522 (43.4%) were women. The random-effects summary EDD was -0.053 (95% CI, -0.065 to -0.040), indicating that women were underenrolled by 5.3 percentage points. This disparity persisted across virtually all geographic regions, intervention types, and stroke types, apart from subarachnoid hemorrhage (0.117 [95% CI, 0.084 to 0.150]). When subarachnoid hemorrhage trials were excluded, the summary EDD was -0.067 (95% CI, -0.078 to -0.057). In the multivariable metaregression analysis, an upper age limit of 80 years as an eligibility criterion was associated with a 6-percentage point decrease in the enrollment of women.
Conclusions and relevance: Further research is needed to understand the causes of the underenrollment of women in acute stroke trials. However, to maximize representation, investigators should avoid imposing age limits on enrollment.
Conflict of interest statement
Figures

Comment in
-
Women in Stroke Trials-A Tale of Perpetual Inequity in Cardiovascular Research.JAMA Neurol. 2021 Jun 1;78(6):654-656. doi: 10.1001/jamaneurol.2021.0624. JAMA Neurol. 2021. PMID: 33900364 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

