Sporulation in solventogenic and acetogenic clostridia
- PMID: 33900426
- PMCID: PMC8102284
- DOI: 10.1007/s00253-021-11289-9
Sporulation in solventogenic and acetogenic clostridia
Abstract
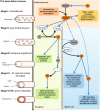
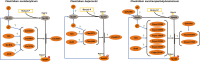
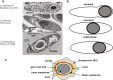
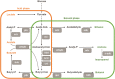
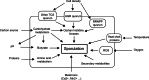
The Clostridium genus harbors compelling organisms for biotechnological production processes; while acetogenic clostridia can fix C1-compounds to produce acetate and ethanol, solventogenic clostridia can utilize a wide range of carbon sources to produce commercially valuable carboxylic acids, alcohols, and ketones by fermentation. Despite their potential, the conversion by these bacteria of carbohydrates or C1 compounds to alcohols is not cost-effective enough to result in economically viable processes. Engineering solventogenic clostridia by impairing sporulation is one of the investigated approaches to improve solvent productivity. Sporulation is a cell differentiation process triggered in bacteria in response to exposure to environmental stressors. The generated spores are metabolically inactive but resistant to harsh conditions (UV, chemicals, heat, oxygen). In Firmicutes, sporulation has been mainly studied in bacilli and pathogenic clostridia, and our knowledge of sporulation in solvent-producing or acetogenic clostridia is limited. Still, sporulation is an integral part of the cellular physiology of clostridia; thus, understanding the regulation of sporulation and its connection to solvent production may give clues to improve the performance of solventogenic clostridia. This review aims to provide an overview of the triggers, characteristics, and regulatory mechanism of sporulation in solventogenic clostridia. Those are further compared to the current knowledge on sporulation in the industrially relevant acetogenic clostridia. Finally, the potential applications of spores for process improvement are discussed.Key Points• The regulatory network governing sporulation initiation varies in solventogenic clostridia.• Media composition and cell density are the main triggers of sporulation.• Spores can be used to improve the fermentation process.
Keywords: ABE production; Acetogens; Quorum sensing; Sigma factors; Solventogenic clostridia; Sporulation.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Abrini J, Naveau H, Nyns E-J. Clostridium autoethanogenum, sp. nov., an anaerobic bacterium that produces ethanol from carbon monoxide. Arch Microbiol. 1994;161(4):345–351. doi: 10.1007/BF00303591. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

